Poster
Efficient, Large-Scale Virus-Like Particle Manufacturing for Gene Editing by a GMP-Compliant Flow Electroporation Platform
Abstract
Virus-like particles (VLPs) are non-infectious, virally-derived nanoparticles that lack a viral genome. Upon packaging a CRISPR ribonucleoprotein complex (RNP), VLPs can target and transiently deliver cargo for genome editing in vivo to specific cell and tissue types. They are safer than traditional viral vectors as they are incapable of genomic integration. Scaling up VLP production can be cumbersome and time-consuming, requiring several rounds of optimization to transition from research to clinical scale. Additionally, consistency and reproducibility between batches is a major concern when relying on chemical methods of VLP production.
Here, we utilized the MaxCyte ExPERT GTx®, a GMP-compliant electroporation instrument, to manufacture clinical-scale VLPs in adherent and suspension HEK cells, packaged with CRISPR-Cas9 or adenine base editor RNPs, for genome editing in primary human cells.
We found that electroporation consistently produced high yields of functional VLPs with an optimized electroporation workflow. We also demonstrated effective gene editing at several therapeutically relevant loci in primary hematopoietic cells, such as B2M and PD-1. Furthermore, the production of VLPs using electroporation exhibited favorable production kinetics compared to other transfection methods, enabling a one-day manufacturing process. Finally, we highlight the scalability of VLP production across a 400-fold volume range with minimal reoptimization, transfecting over one billion cells per production.
In summary, our results show that MaxCyte’s Flow Electroporation® technology is a viable means for consistent, efficient and scalable manufacturing of VLPs for gene editing applications and has high promise to address the needs of future clinical and commercial manufacturing.
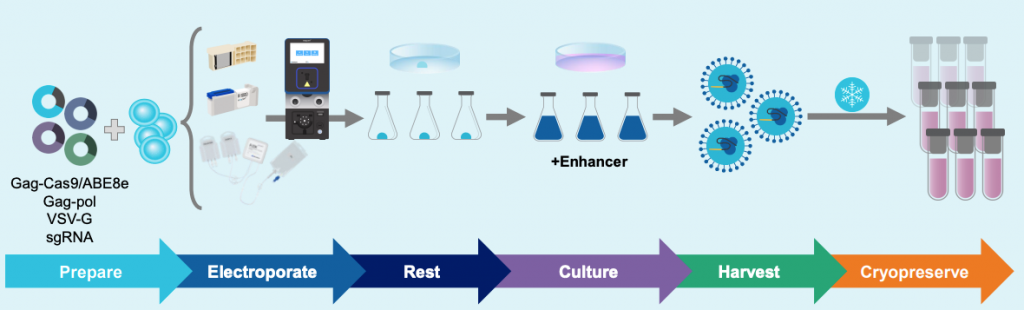
Experimental workflow for VLP production & assessment of yields
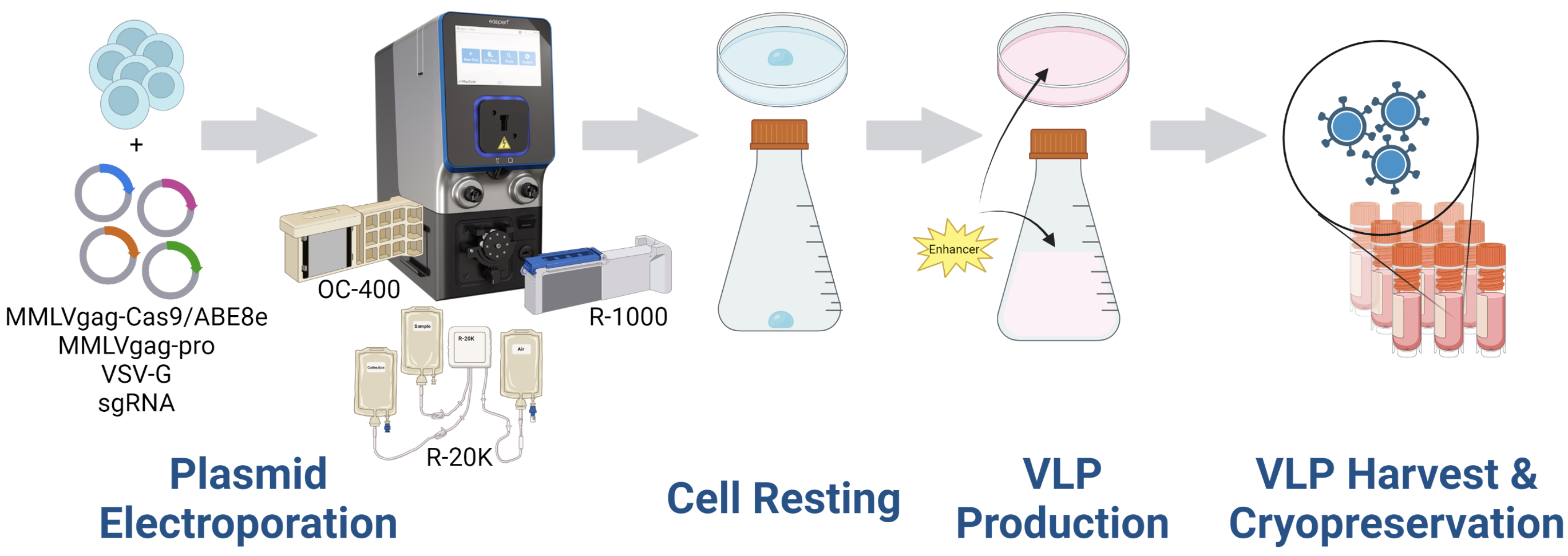
A) Electroporation workflow
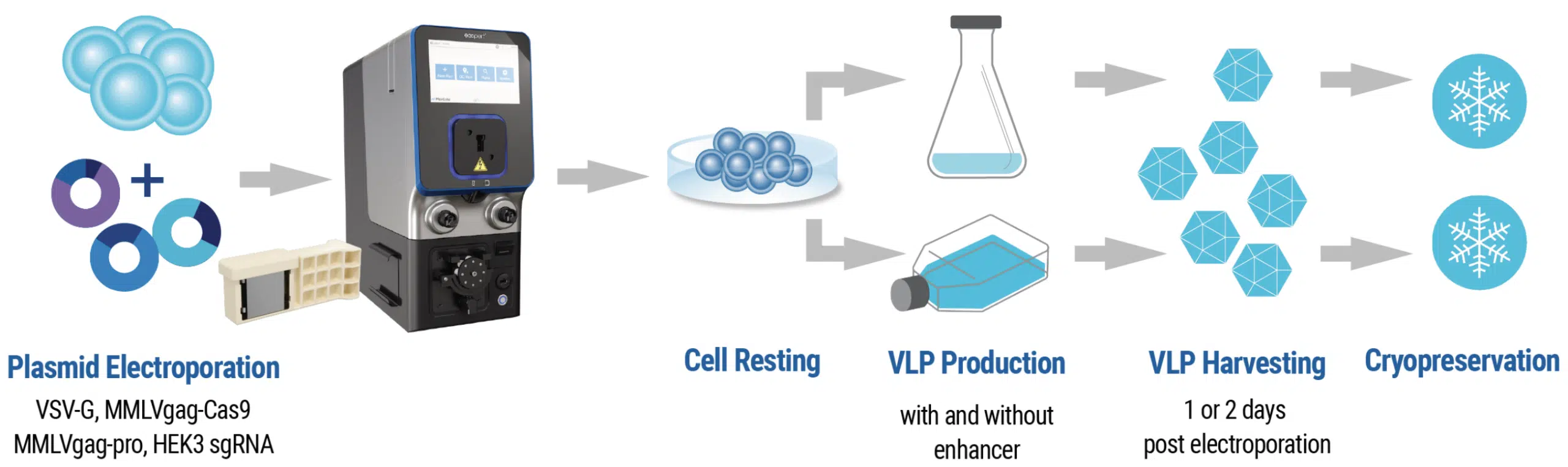
B) Transduction and analysis workflow
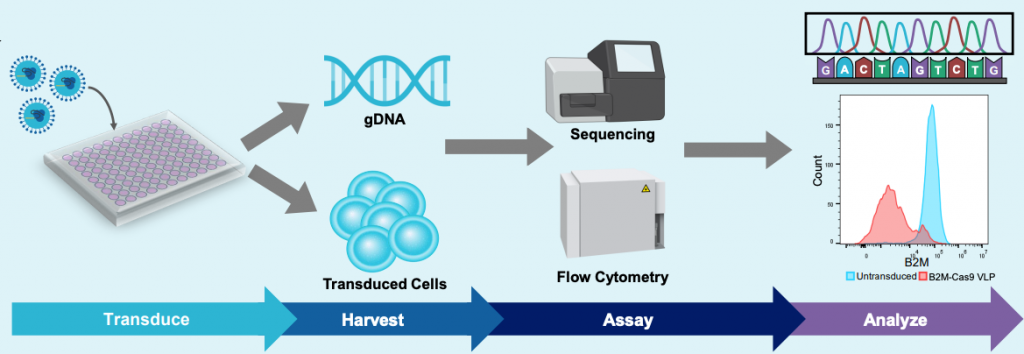
Figure 1: A) HEK293 cells were resuspended in MaxCyte electroporation buffer with a fixed-ratio plasmid DNA mix. Cells were electroporated in a static or flow processing assembly (PA) with a pre-loaded, optimized program. Electroporated cells were rested briefly in the final culture vessel and then supplemented with production media and enhancer. Culture media was harvested 24- or 48- hours post electroporation and VLPs were precipitated from the clarified supernatant. VLPs were then resuspended in 0.05-0.1 volumes PBS and stored in aliquots at -80°C. B) HEK293T or activated T cells were seeded in multi-well plates and transduced with the indicated volume of concentrated VLPs diluted in PBS. Three to five days post transduction, cells are harvested and either lysed for genomic DNA (gDNA) extraction or stained with fluorescently conjugated antibodies for flow cytometry. gDNA is PCR amplified with locus-specific primers and subject to Sanger sequencing and editing analysis (ICE, Synthego; EditR, Kluesner M et al. 2018. doi: 10.1089/crispr.2018.0014. PMID: 31021262).
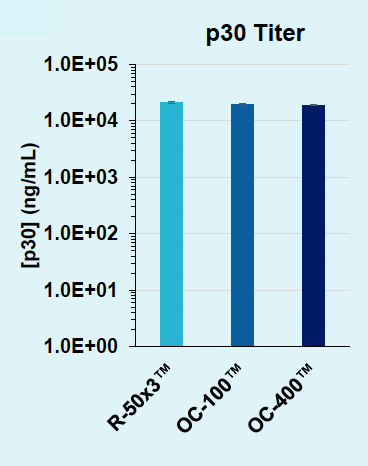
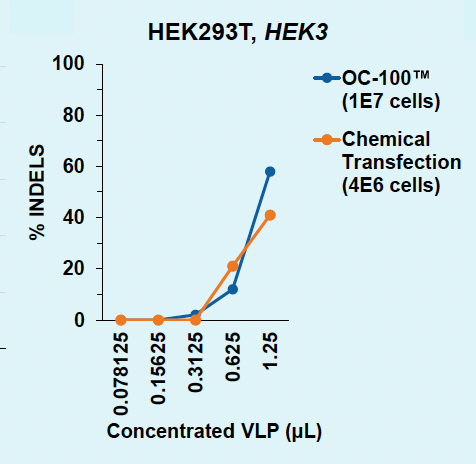
ExPERT™ platform enables scalable VLP production in adherent HEK293 cells
HEK3-targeting Cas9-loaded VLPs were produced in adherent HEK293 cells and harvested from the supernatant one or two days post transfection. VLPs were titrated onto HEK293T or assayed by ELISA. Indels were analyzed via Synthego ICE following genomic locus amplification and Sanger sequencing.
A) Day 1: HEK293T, HEK3

B) Day 1: p30 titer
C) Days 1v2: HEK293T, HEK3
D) Days 1v2: p30 titer
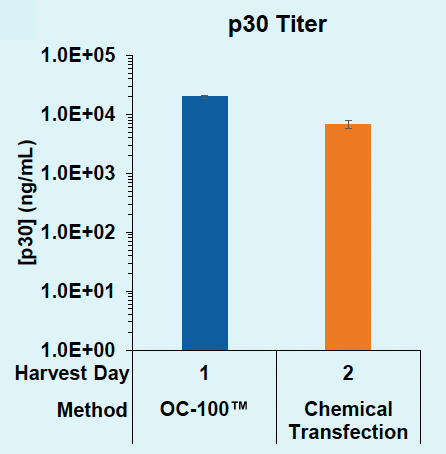
Figure 2: A) Functional VLPs are efficiently produced in adherent HEK293 in one day following electroporation, at every cell number tested. B) High VLP titers are consistently produced from adherent HEK293 cells in one day. C) Electroporation enables a one-day production timeline for efficient VLP production, whereas chemical transfection requires a two-day production. D) Electroporation results in higher physical VLP titers within one day compared to chemical transfection on day two.
ExPERT platform enables scalable VLP production in suspension HEK293 cells
A) Day 1: HEK293T, HEK3

B) Day 1: p30 titer
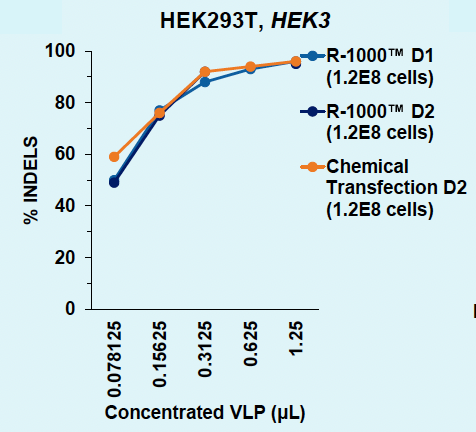
C) Day 1 versus 2: HEK293T, HEK3
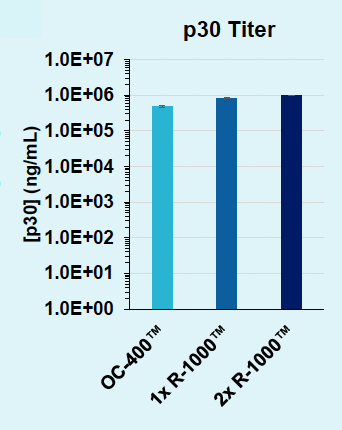
D) Day 1 versus 2: Titer
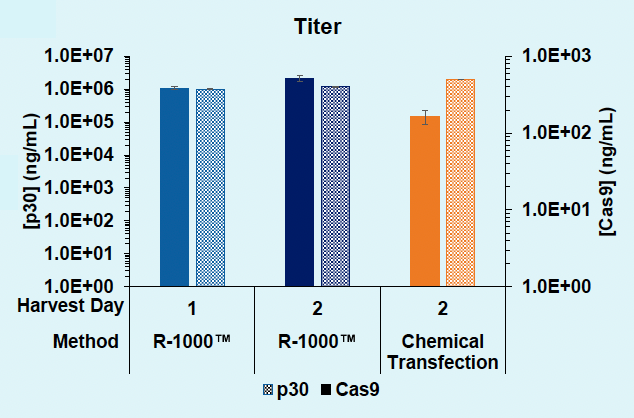
Figure 3: A) Functional VLPs are efficiently produced in suspension HEK293 in one day following electroporation, at every cell number tested. B) High VLP titers are consistently obtained from suspension HEK293 cells in one day. C) Electroporation enables a one- or two- day production timeline, whereas chemical transfection requires a minimum of two days for efficient VLP production in suspension HEK293 cells. D) Electroporation results in comparable physical VLP titers and improved Cas9 titer compared to chemical transfection.
Electroporation produces high-titer VLPs sufficient for primary immune cell editing
B2M-targeting Cas9-loaded VLPs were produced in suspension HEK293 cells using the R-1000 PA™ and harvested from the supernatant one day post electroporation. T cells were isolated from frozen PBMCs and activated for 48 hours prior to transduction. Activated cells were seeded in RetroNectin®-coated plates and transduced for two days, followed by expansion and re-transduction with the same volume indicated.
A) Percentages of B2M INDELS in HEK293T
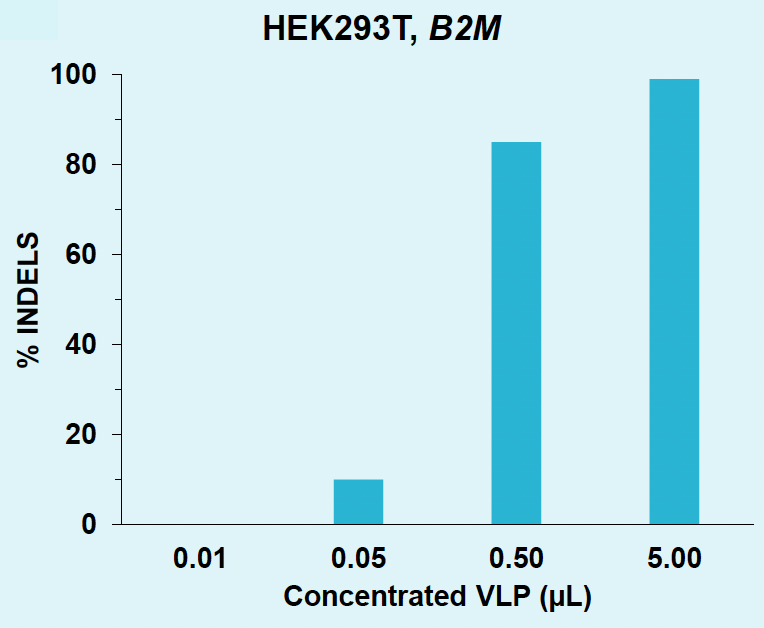
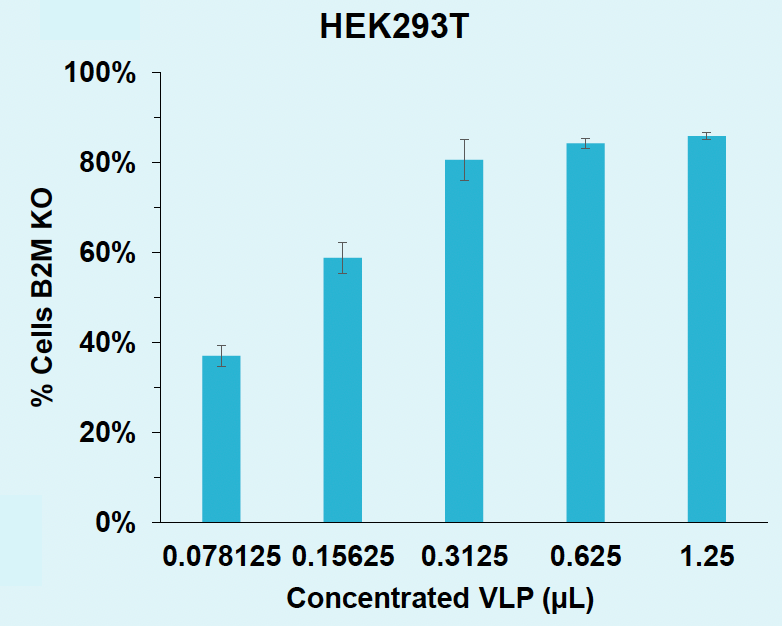
B) B2M knockout rates in HEK293T
C) B2M knockout rates in T cells
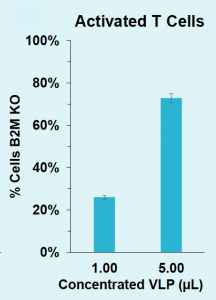
Figure 4: A) B2M locus is efficiently edited in HEK293T with VLPs produced in suspension HEK293. B) B2M in HEK293T is efficiently knocked out with VLPs produced in suspension HEK293. C) B2M in activated T cells is efficiently knocked out with VLPs produced in suspension HEK293.
Electroporation facilitates high incorporation of ABE8e into VLPs
HEK3- or B2M- targeting ABE8e-loaded VLPs were produced in suspension HEK293 cells using the R-1000™ PA and harvested from the supernatant one day post EP. Base edits were analyzed via EditR following genomic locus amplification and Sanger sequencing.
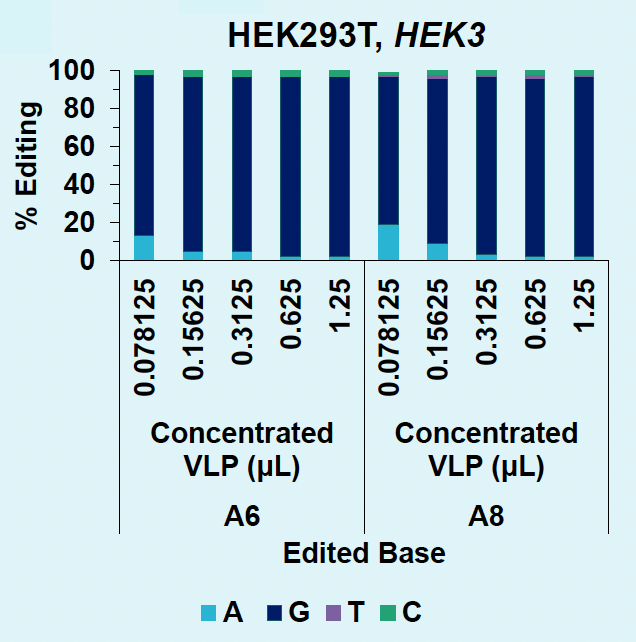
A) Editing rates for HEK3-targeting VLPs
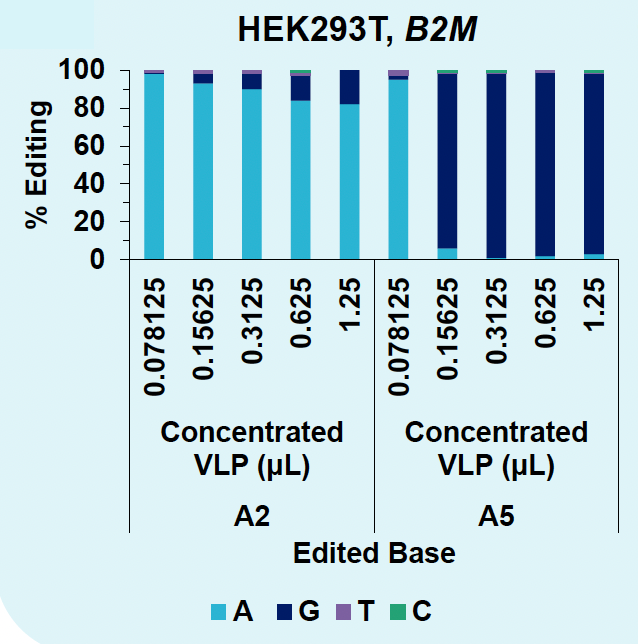
B) Editing rates for B2M-targeting VLPs
C) Rates of B2M knockout in HEK293T
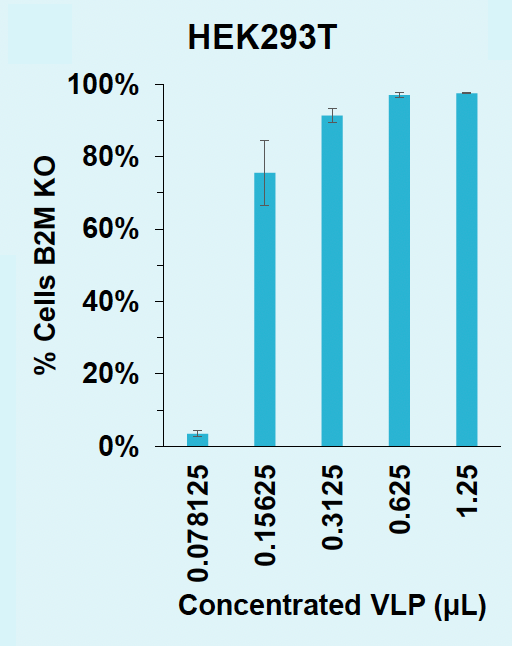
D) Rates of B2M knockout in T cells

Figure 5: A) HEK3 locus in HEK293T is efficiently edited with low doses of concentrated VLPs. B) A5 within the B2M locus in HEK293T is efficiently edited to G with concentrated VLPs. C) ABE8e-VLPs efficiently disrupt B2M surface expression in HEK293T. D) ABE8e-VLPs efficiently disrupt B2M surface expression in activated T cells.
Large-scale VLP production requires minimal re-optimization on the ExPERT Flow Electroporation® platform
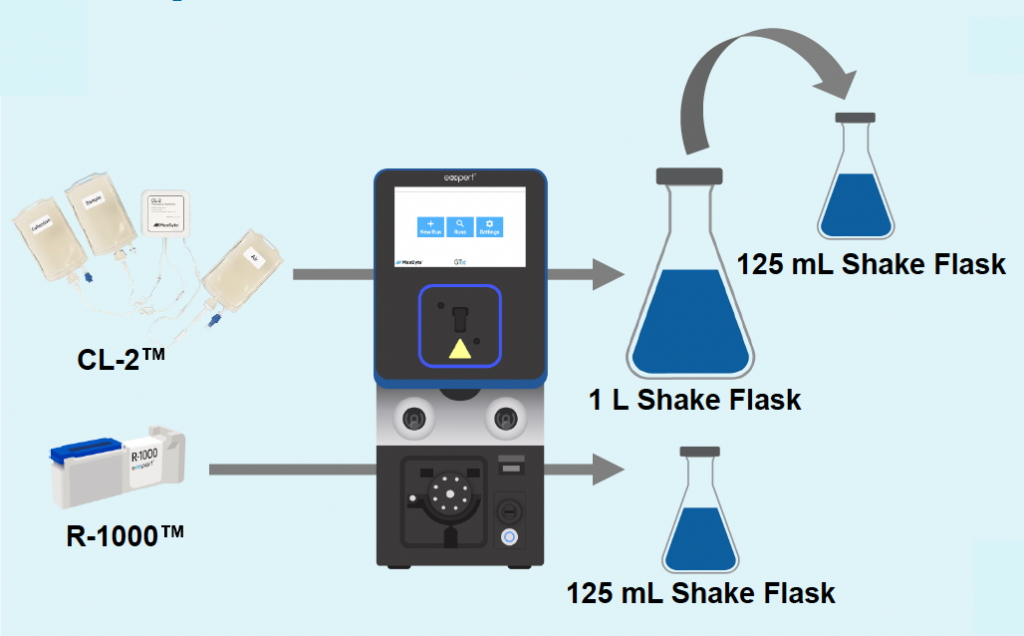
A) Re-optimization workflow
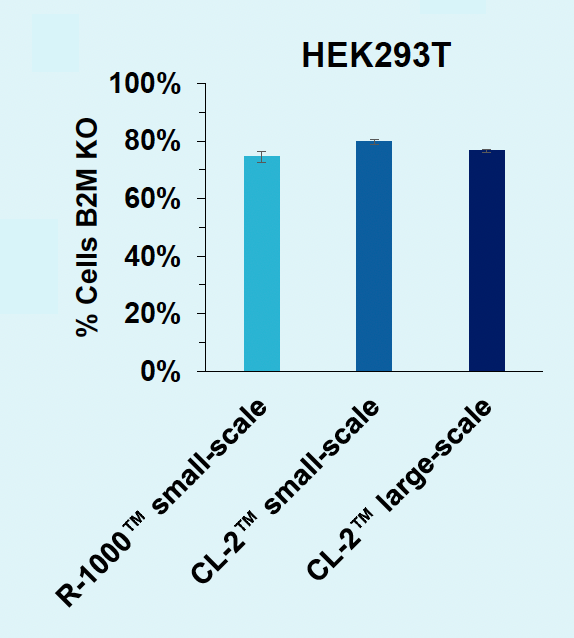
B) B2M knockout for scale-up
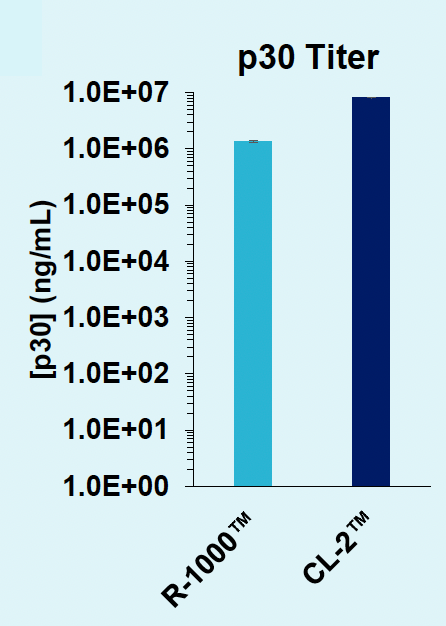
C) Titer contrast between PAs
Figure 6: A) B2M-targeting Cas9-loaded VLPs were produced in suspension HEK293 cells, in either an R-1000 static PA (1 x 108 cells) or CL-2™ flow PA (1.5 x 109 cells). After cells were rested and supplemented with production media, an aliquot of cells was removed from the 1 L shake flask (CL-2) and cultured in a 125 mL shake flask to match small-scale production (R-1000) culture conditions. B) VLPs produced at large scale can edit the B2M locus in HEK293T cells with the same potency (0.625 μL concentrated VLP) as those produced at small scale. C) VLP production can be scaled up for manufacturing without the need for additional process optimizations.
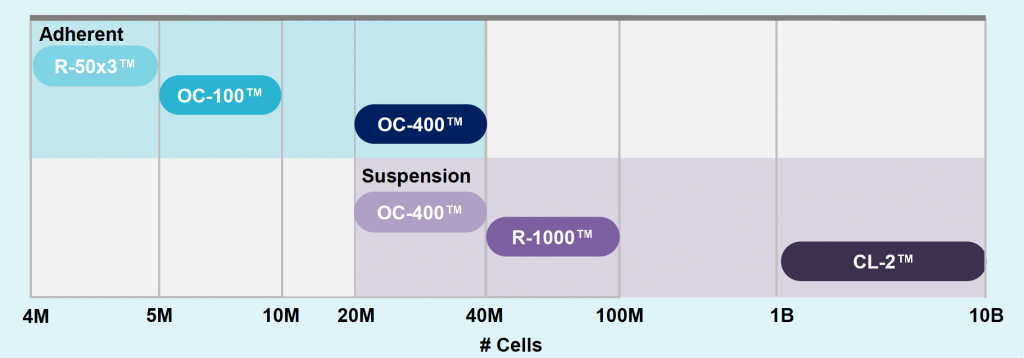
ExPERT platform enables 400-fold volume scalability
Summary
- The MaxCyte ExPERT Flow Electroporation® platform enables VLP production, in both adherent and suspension HEK293 cells.
- Incorporation of a variety of RNPs into VLPs via electroporation compares favorably to chemical transfection.
- VLPs produced via electroporation are potent, directing high-efficiency gene editing at low doses, and can be achieved within a one-day production timeline.
- VLP production is scalable, from 5 million to 10 billion cells, without the need for additional process development, maintaining a titer comparable to small scale.
References
- An M, et al. Nat Biotechnol. 2024. doi: 10.1038/s41587-023-02078-y. PubMed PMID: 38191664
- Banskota S, et al. Cell. 2022;185(2):250-65.e16. doi: 10.1016/j.cell.2021.12.021. PubMed PMID: 35021064
- Hamilton JR, et al. Cell Rep. 2021;35(9):109207 doi:10.1016/j.celrep.2021.109207. PubMed PMID: 34077734.
- Kluesner MG, et al. Crispr J. 2018;1(3):239-50. doi: 10.1089/crispr.2018.0014. PubMed PMID: 31021262
- Raguram A, Banskota S, Liu DR. Cell. 2022;185(15):2806-27. doi: 10.1016/j.cell.2022.03.045. PubMed PMID: 35798006