Scientific Brief
mRNA CAR NK Cells as Potential Anti-Sarcoma, Solid Tumor Therapy
Background
Sarcomas are a group of aggressive cancers with a particularly poor prognosis in cases of advanced disease; new therapeutic options are desperately needed.1 Despite the commercialization of multiple cell therapies for treating various hematological disorders, immunotherapies such as CAR T cells have not had overwhelming success in treating solid tumors.
A greater appreciation of the innate anti-tumor role of NK cells and their compatibility with allogeneic transfer are driving efforts to develop CAR NK therapies for solid tumors. High NK cell infiltration in sarcomas is reportedly associated with improved survival, making a CAR NK treatment approach particularly attractive.2 Various gene editing and transfection methods have been used for CAR NK creation.3 Among these, CAR mRNA electroporation avoids potential challenges with genetic engineering and viral transduction in NK cells.
Ephrin type-A receptor-2 (EphA2) is highly expressed in pediatric sarcomas. EhpA2 plays a critical role in developing embryos, but expression in adults is mainly restricted to proliferating epithelial cells. In preclinical studies, anti-EphA2 CAR cells (T and NK) have demonstrated efficacy against various carcinoma models, including sarcomas.
Here, the authors developed anti-EphA2 CAR NK cells and evaluated their ability to target in vitro and in vivo models of pediatric sarcoma.2
MaxCyte® workflow for CAR NK production
NK-92, or human primary NK cells, were resuspended in MaxCyte electroporation buffer containing RNase inhibitor and anti-EphA2 CAR mRNA (200 μg/mL). Cells were electroporated using the ExPERT STx® and an optimized, pre-loaded NK cell electroporation protocol. After resting, cells were cultured for 24 to 72 hours before downstream analysis and functional evaluation.
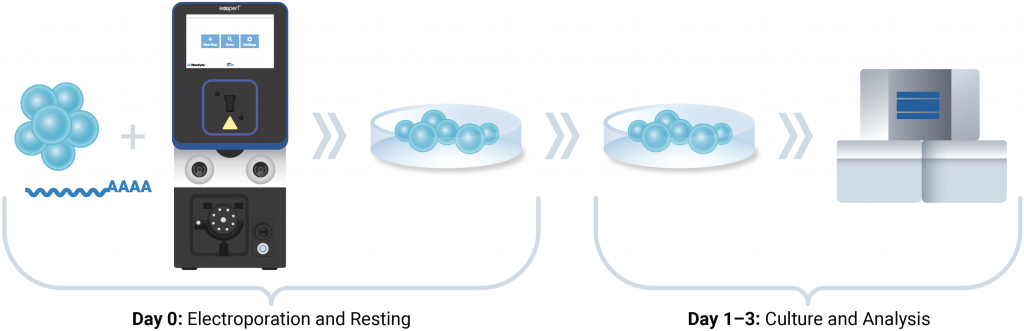
Experimental approach
The optimal method for CAR NK manufacturing was determined by comparing anti-EphA2 CAR mRNA delivery by lipid nanoparticles or MaxCyte electroporation. Anti-EphA2 CAR expression levels were determined by flow cytometry using an anti-CAR linker antibody. For subsequent experiments, CAR NK cells were manufactured by mRNA electroporation of peripheral blood-derived NK cells and NK-92 cells. In vitro cytotoxicity and in vivo efficacy in an osteosarcoma mouse model were tested.
Results
A)
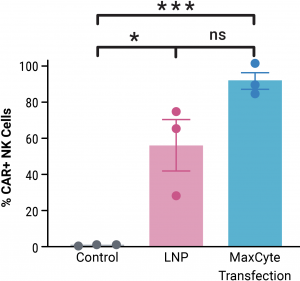
A) Primary peripheral blood-derived NK cells transfected with MaxCyte electroporation showed higher CAR expression than cells transfected with lipid nanoparticles. Three donors were tested. Data are presented as mean ± SEM. Statistical analyses were conducted using unpaired t-test; where ns = not significant; *p < .05; ***p < .001.
B)

B) CAR NK-92 cells showed enhanced in vitro killing activity against EphA2-expressing sarcoma cell lines (MG63, U2-OS, SaOS, A673, RD and RH30) compared with mock-transfected NK-92 cells. Data represent two independent experiments presented as mean ± SE and compared using an unpaired t-test (Holm-Sidak method) at each effector-to-target (E: T) ratio; *p < .05; **p < .01; ****p < .0001.
C)
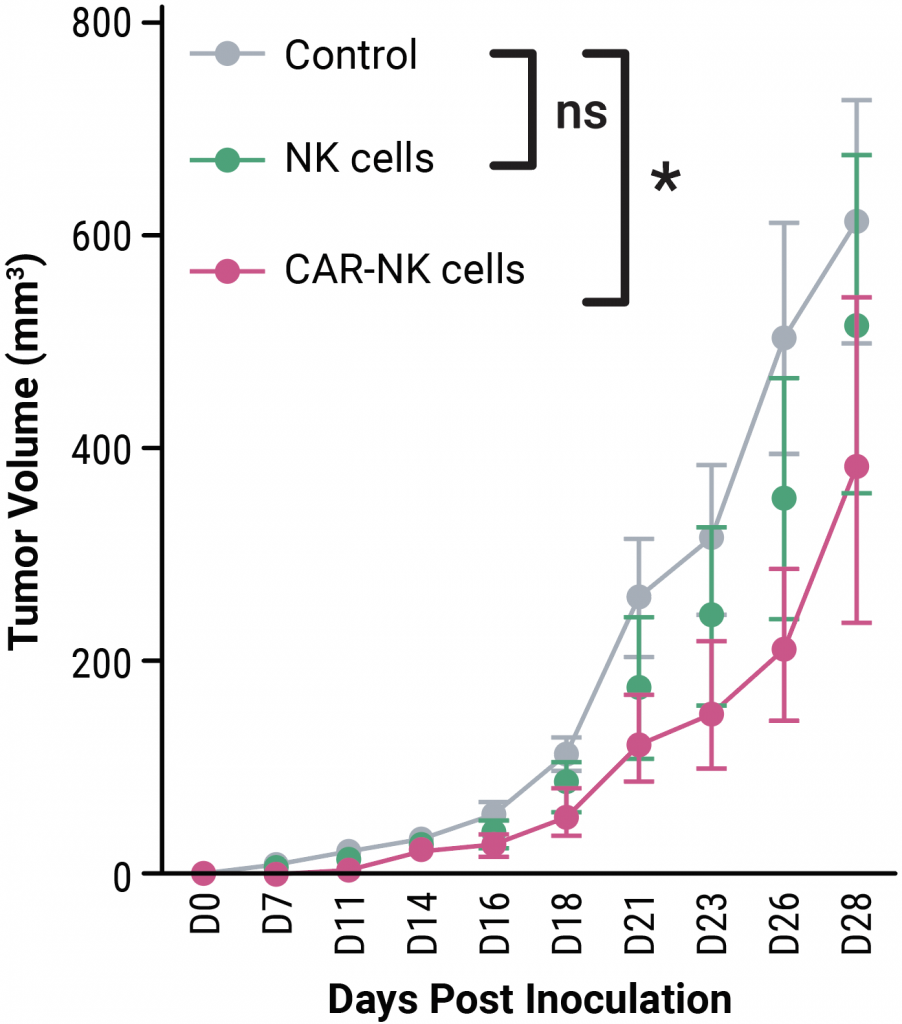
D)

On days four, eight, 11 and 14 following tumor inoculation, MG63 model mice were treated with peripheral blood-derived mock or CAR NK cells or left untreated (PBS or control). C) Tumor growth was significantly restricted in mice treated with CAR NK cells. D) The overall survival rate was higher in mice treated with CAR NK cells. Statistical analyses were performed between groups in (C) by two-way analysis of variance (ANOVA) with Tukey’s multiple comparison test and in (D) by Gehan-Breslow-Wilcoxon test; where ns = not significant, *p < .05. Results were generated from n = 6–7 mice per group per experiment.
Summary
- MaxCyte mRNA electroporation outperformed LNP delivery for anti-EphA2 CAR NK creation from peripheral blood NK cells.
- Anti-EphA2 CAR NK cells effectively targeted sarcoma cells in an in vitro killing assay.
- MG63 sarcoma model mice treated with anti-EphA2 CAR NK cells had reduced tumor burden and prolonged survival.
- MaxCyte’s clinically validated Flow Electroporation® technology can transfect up to 2x1011 cells, enabling the development and manufacturing of allogeneic CAR NK therapies.
References
- Lachota M, Vincenti M, Winiarska, M, et al. Prospects for NK Cell Therapy of Sarcoma. Cancers. 2020, 12, 3719. https://doi.org/10.3390/cancers12123719
- Lam PY, Omer N, Wong JKM, et al. Enhancement of anti-sarcoma immunity by NK cells engineered with mRNA for expression of a EphA2-targeted CAR. Clin Transl Med. 2025; 15:e70140. https://doi.org/10.1002/ctm2.70140
- Gong, Y., Klein Wolterink, R.G.J., Wang, J. et al. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021, 14, 73. https://doi.org/10.1186/s13045-021-01083-5








