Our journey began with a mission to lower risk in cell therapy development and regulatory submission, ensuring safer therapies reach patients faster. To achieve this, we developed cutting-edge on-target and off-target assessment assays, providing unparalleled insights into genomic integrity. These innovations drive precision medicine forward, empowering safer, more effective therapeutic advancements.

A history of innovation driving safer cell and gene therapies
2013
Birth of CRISPR biotech therapeutics companies
First CRISPR-based gene editing therapeutics companies are founded.
2013
First CRISPR off-target publications
Of which, Fu, Y. et. al. “High-frequency off-target mutagenesis induced by CRISPR-cas nucleases in human cells ” published in Nature Biotechnology.
2014
GUIDE-Seq launch
GUIDE-seq developed in Keith Joung’s Lab to identify off-target editing.
2018
First CRISPR clinical trial
First CRISPR-based gene editing human clinical trial starts.
2020
Founding
SeQure founded to identify and manage off-target risks for biopharma.
2022
New FDA guidance
FDA published draft guidance “Human Gene Therapy Products Incorporating Human Genome Editing."
2025
Joined MaxCyte
MaxCyte acquires SeQure to enhance its best-in-class tools and services portfolio, reflecting its ongoing commitment to become a premier cell engineering solutions provider.
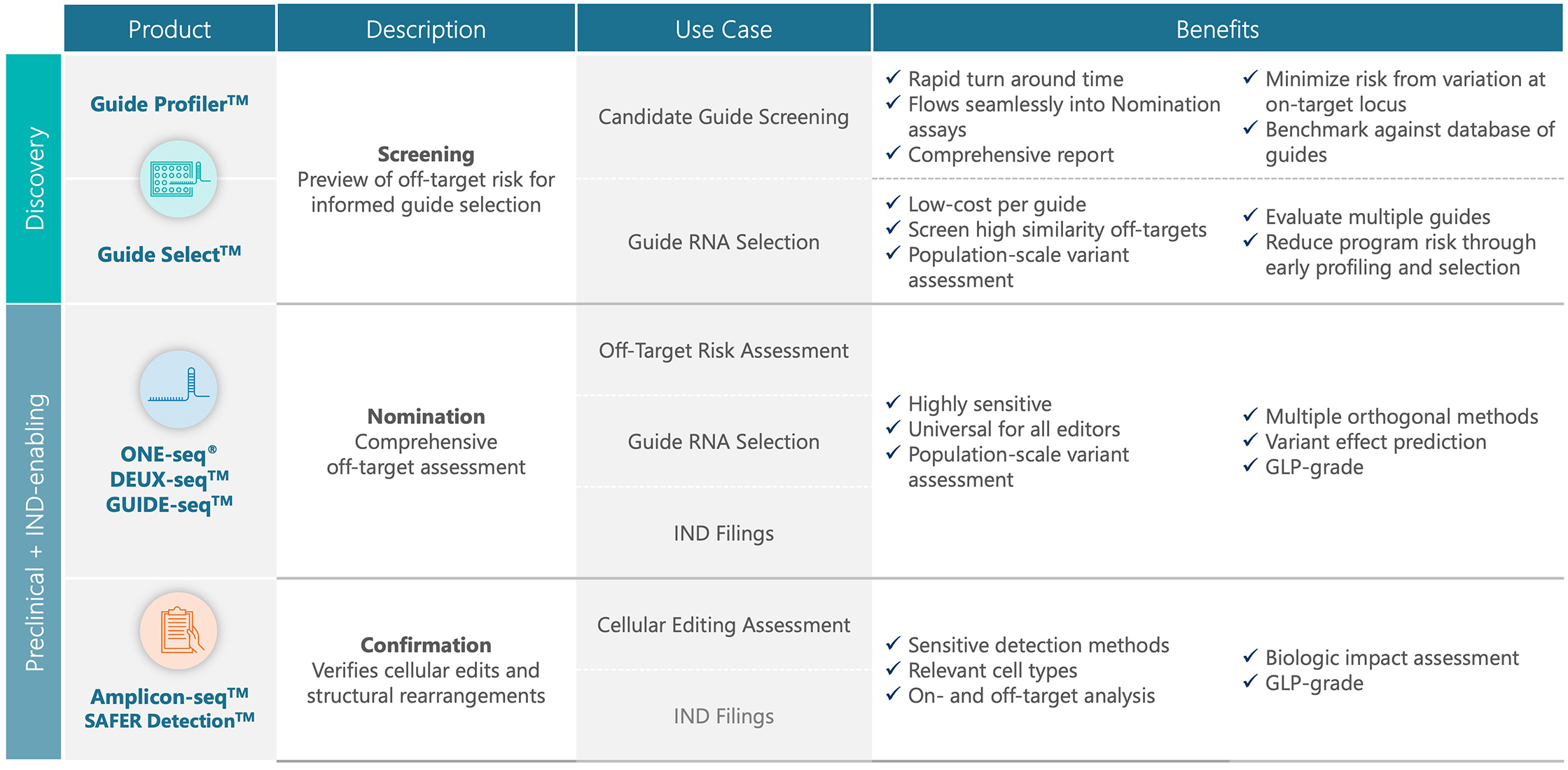
Learn more about our comprehensive suite of assays spanning from early discovery through pre-clinical and clinical development.
Have questions?








