Enhancing Non-Viral CAR T Manufacturing with MaxCyte Electroporation and Akadeum Microbubble T Cell Isolation
Cell & Gene Tech Expo: Cell Therapy Solutions
Virtual session
October 22, 2025
Efficient and reliable non-viral engineering workflows depend on both a robust electroporation platform and healthy upstream cell populations. In this webinar, MaxCyte will showcase how pairing our clinically validated electroporation technology with Akadeum’s gentle microbubble-based cell isolation can strengthen outcomes in engineered T cell workflows.
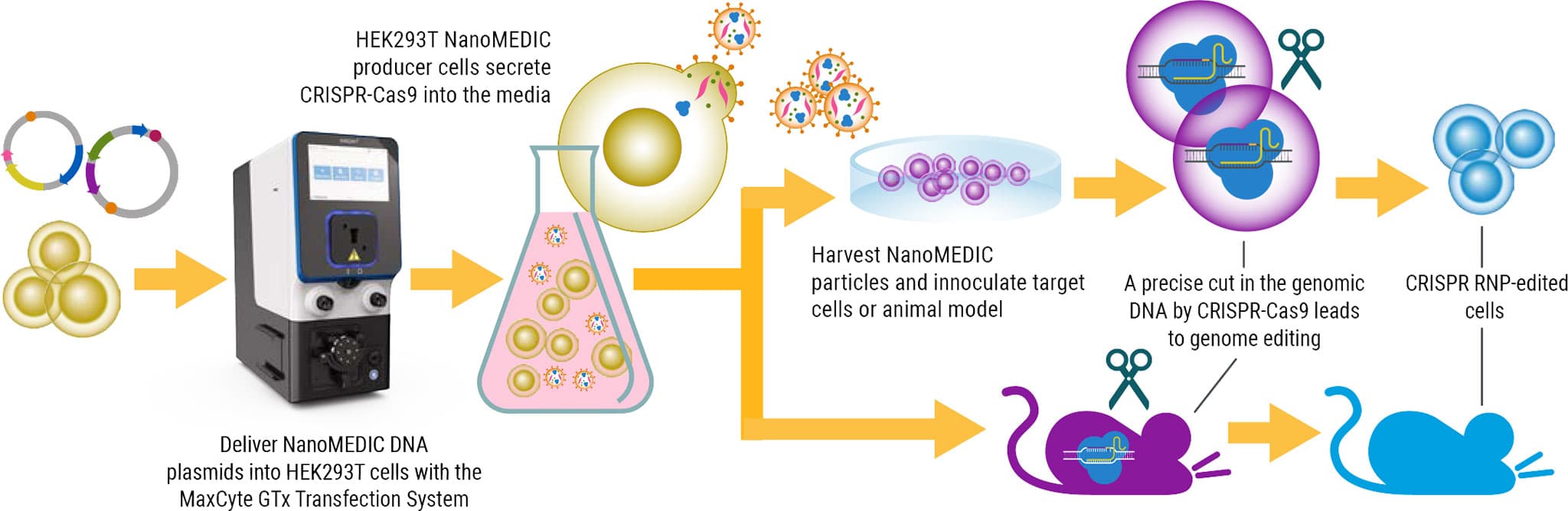
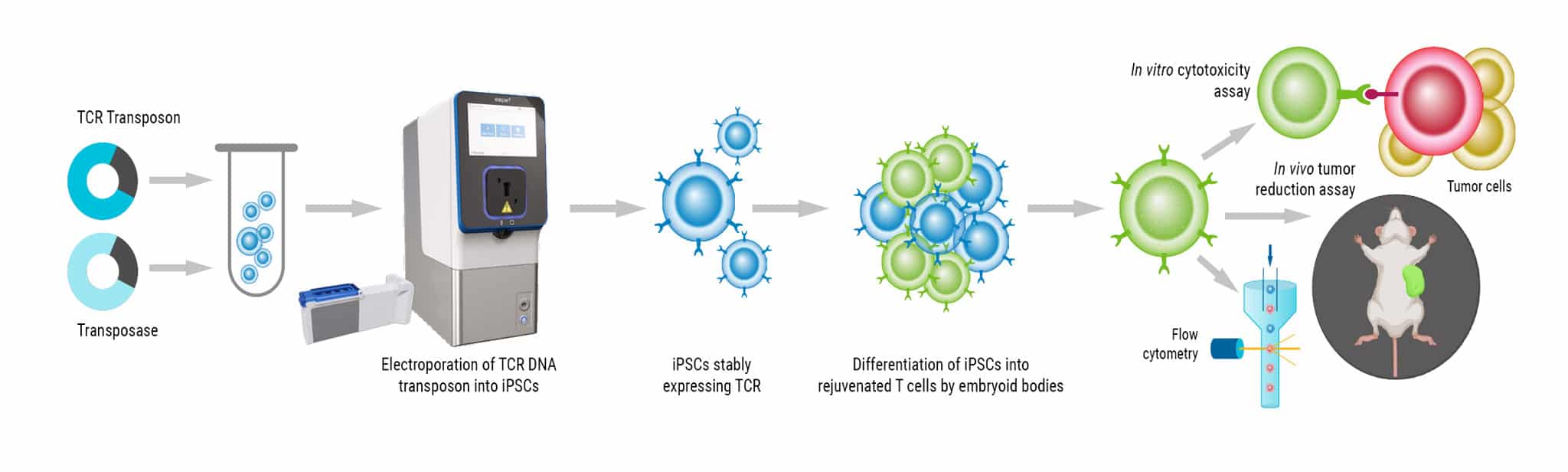
We will highlight experiments using DNA and mRNA transient expression, as well as a representative CRISPR-based CD19 CAR knock-in workflow that mirrors a standard non-viral CAR T manufacturing process. Across these applications, integrating Akadeum’s microbubble isolation upstream of MaxCyte electroporation improved post-engineering outcomes, including higher cell numbers, greater viability, and superior transfection efficiency compared to conventional magnetic separation methods.
Attendees will gain practical insights into how combining Akadeum’s innovative microbubble technology with MaxCyte’s proven electroporation platform can deliver more reproducible results, streamline workflows, and ultimately accelerate the advancement of engineered T cell therapies.
Key Learning Objectives:
- Understand how upstream T cell isolation impacts performance and consistency in MaxCyte electroporation workflows.
- Learn how Akadeum’s microbubble technology compares to magnetic separation for isolating naïve and activated T cells.
- Explore how integrating microbubble isolation with MaxCyte electroporation improves cell yield, viability, and transfection efficiency in non-viral CAR T workflows.
Speakers


Relevant Resources
Explore additional materials to learn how MaxCyte and Akadeum are streamlining non-viral CAR T manufacturing workflows.
Have more questions?
Send your question to one of our cell engineering experts.