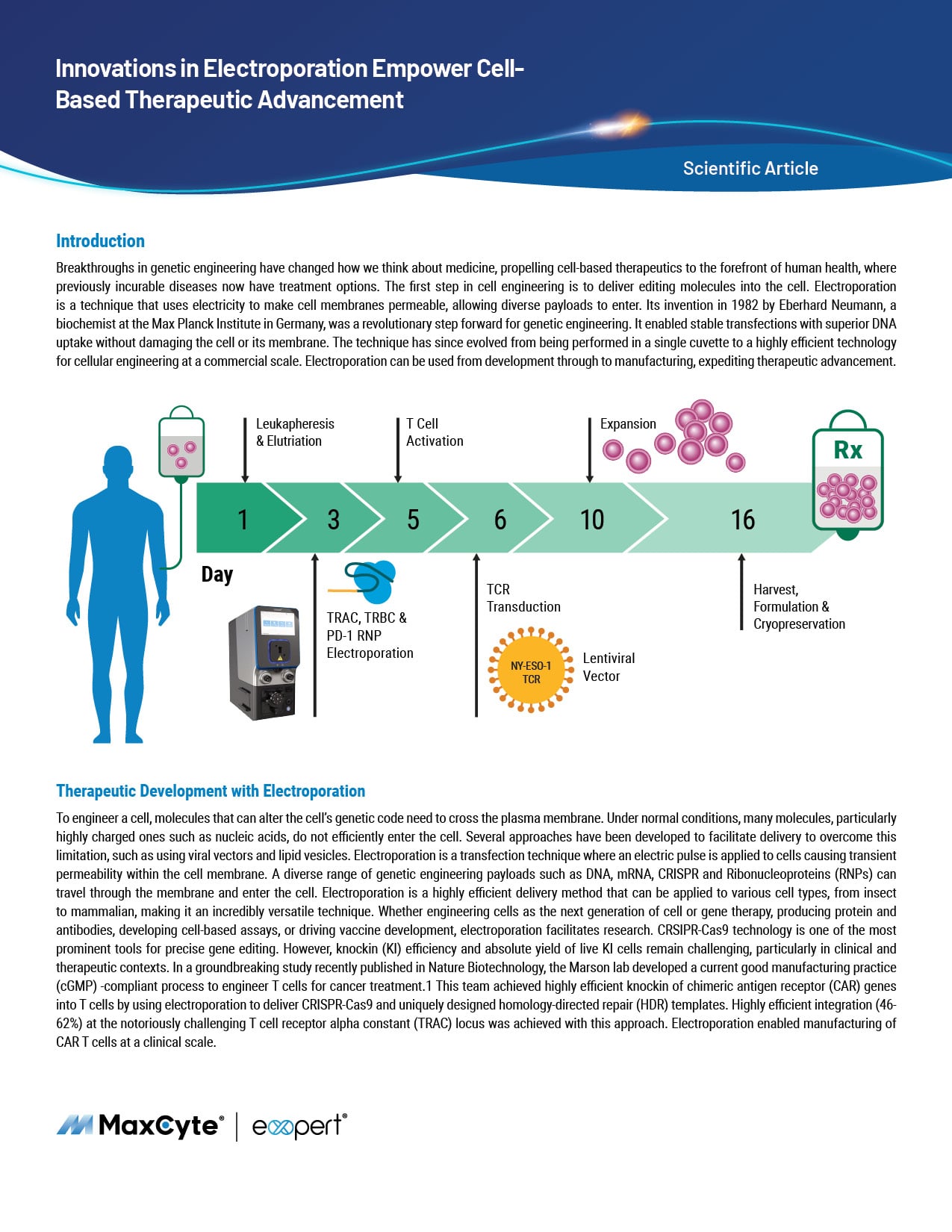
MaxCyte® Electroporation Helps Engineer Difficult-to-Transfect Immune Cells
5th International Conference on Lymphocyte Engineering (ICLE) Poster Presentation
Munich, Germany
February 20-22, 2025
MaxCyte scientist Ashley Strickland-Dietz presents her poster from the 2025 International Conference on Lymphocyte Engineering
Abstract
Since their inception, cell-based therapies have emerged as promising treatments for a wide range of diseases. Immune cells such as T cells, NK cells and macrophages are being used to treat various cancers, autoimmune disorders and degenerative diseases. To engineer these cells for improved efficacy and safety, biomolecules and other genome-editing tools must be delivered into these difficult-to-transfect cells. To this end, MaxCyte® has developed optimized cell engineering workflows using the ExPERT™ electroporation platform that enable highly efficient delivery of molecules, such as RNA, DNA and CRISPR-Cas nucleases, into a variety of cell types. Here, we demonstrate that these workflows can be used to engineer primary human immune cells to express tumor-targeting receptors while maintaining high cell viabilities and functionality. In particular, MaxCyte enabled transient and stable expression of CARs/TCRs in T cells, NK cells and macrophages through high-efficiency transfection of mRNA, DNA encoding transposons/transposases, or CRISPR ribonucleoproteins (RNPs) and homology-directed repair (HDR) templates into these hard-to-transfect cells. In addition, these workflows seamlessly scaled up, allowing these cells to be engineered at therapeutically relevant scales.
Key takeaways
- Efficient workflow: The ExPERT GTx® facilitated transfection efficiencies of nearly 80 percent higher in T cells, NK cells and macrophages within one day of electroporation.
- Versatility: MaxCyte enables stable integration of transient CAR and TCR expression, tumor-targeting molecules, transposon systems and CARs into a range of cell types.
- Scalability: For NK cells, optimized electroporation conditions were scaled from 1.3 million cells to 2.5 billion cells with no drop in transfection efficiency or viability. For T cells, conditions optimized at 120 million cells were used to engineer 3.9 billion cells without the need for re-optimization and with no impact on cell viabilities
- Safety and functionality: Cells engineered using electroporation remained viable and functional both in vitro and in vivo.
Watch presentation on immune cell transfection
View the poster that established an optimized process for engineering hard-to-transfect immune cells using MaxCyte's electroporation technology
Have more questions?
Send your question to one of our cell engineering experts.
Presenter

Related resources
Poster presentation transcript
Hello, my name is Ashley Strickland-Dietz, and I am the immunology scientist at MaxCyte. Today I will be presenting case studies highlighting MaxCyte’s capability to enable highly efficient engineering of difficult-to-transfect immune cells, which were presented as a poster at the 2025 International Conference on Lymphocyte Engineering in Munich, Germany.
Since their inception, cell-based therapies have emerged as promising treatments for a wide range of diseases. Immune cells such as T cells, NK cells and macrophages are being used to treat various cancers, autoimmune disorders and degenerative diseases. To engineer these cells for improved efficacy and safety, biomolecules and other genome-editing tools must be delivered into these difficult-to-transfect cells. With this in mind, MaxCyte has developed optimized cell engineering workflows using the ExPERT electroporation platform that enable highly efficient delivery of molecules such as RNA, DNA and CRISPR-Cas nucleases into a variety of different cell types. Here, we share several case studies that demonstrate MaxCyte’s capabilities to enable immune cell engineering while maintaining high cell viability and functionality.
To begin, we demonstrate that MaxCyte electroporation can be used to achieve transient CAR expression in activated T cells, NK cells and monocyte-derived macrophages through mRNA transfection. In particular, the ExPERT GTx enabled transfection efficiencies of nearly 80 percent higher in all three cell types by 24 hours post-electroporation, with no impact on cell viability. In the case of T cells, CAR expression was sustained for up to seven days. For NK cells, optimized electroporation conditions were scaled from 1.3 million cells to 2.5 billion cells with no drop in transfection efficiency or viability. And for macrophages, CAR expression could be improved by increasing the concentration of CAR mRNA electroporated into cells.
Similarly, MaxCyte can be used to engineer cells with mRNA encoding TCRs. For the generation of TCR T cells for the treatment of hepatocellular carcinoma, activated T cells were transfected with TCR mRNA using the ExPERT GTx. Engineering conditions were optimized at a smaller scale of 120 million cells and were used to engineer T cells at a larger scale of 3.9 billion cells without the need for re-optimization. At both scales, TCR expression was sustained for up to 48 hours in approximately 90 percent of T cells, with no impact on cell viabilities. These cells were then divided into three groups and frozen at different times post-electroporation, thawed and reexamined. TCR expression and viabilities were maintained following freezing and thawing. Moreover, these cells remained functional and produced similar amounts of interferon gamma [IFN-γ] in response to target cells, as did fresh TCR T cells.
In addition to transient CAR and TCR expression, MaxCyte also enables stable integration of tumor-targeting molecules into various cell types. In this study, CD70 CAR NK cells for the treatment of hematological and solid malignancies were engineered using the TcBuster transposon system. Because NK cells expanded IL-2 and feeder cells were found to upregulate their expression of CD70, this group also needed to knock out CD70 expression in NK cells to prevent fratricide by CD70 CAR-positive cells.
Using the ExPERT ATx, this group was able to co-electroporate Cas9 mRNA and single-guide RNA, targeting CD70 alongside CD70 CAR transposon DNA and TcBuster transposase mRNA in order to simultaneously knock out CD70 and integrate a CD70 CAR. This single-step process prevented fratricide and resulted in approximately 50-80 percent CAR integration along with greater than 80 percent knockout of CD70. These CAR NK cells were functional and underwent degranulation, produced pro-inflammatory cytokines such as interferon gamma and TNF-α, and killed target cells in an antigen-specific manner.
Likewise, MaxCyte can also be used to deliver different transposon systems into different cell types. Here, the ExPERT GTx was used to engineer CAR T cells for the treatment of AML [acute myeloid leukemia] via delivery of minicircle transposon DNA and Sleeping Beauty transposase mRNA. MaxCyte electroporation enabled high-efficiency transposition in both healthy and patient T cells, and transposition did not impact the ability for these cells to expand. In addition, CAR T cells were functional and eradicated nearly 100 percent of target cells, at effector-to-target ratios as low as 0.6 to 1. More importantly, engineering T cells using the Sleeping Beauty transposon system along with MaxCyte was safe, as both transposase protein and unintegrated minicircle transposon DNA were undetectable within the cells by days 7 and 15 post-electroporation, respectively.
In addition to transposons, stable integration of CARs can also be achieved using CRISPR-Cas systems in MaxCyte. In particular, the ExPERT GTx enabled CRISPR-mediated knock-in of a BCMA CAR at the track locus in activated T cells through the delivery of Cas9 RMPs and single-stranded linear HDR templates containing Cas9 targeting sequences. CAR knock-in was highly efficient and did not impact T cell expansion. Small molecule enhancers further improved CAR knock-in efficiencies from 40 percent to 60 percent. In vitro, these engineered CAR T cells were able to target and kill over 90 percent of target cells at effector-to-target ratios of 1 to 1. In vivo, these BCMA CAR T cells also improved survival in tumor-bearing mice.
Similarly, MaxCyte also enabled CD19 CAR knock-in at the track locus in activated T cells through the delivery of Cas9 RMPs and double-stranded linear HDR templates. CAR knock-in did not impair the ability for these cells to expand while track knockout efficiencies were over 90 percent and knock-in efficiencies were greater than 60 percent. The addition of a small molecule enhancer could further improve knock-in efficiencies to over 80 percent. Together, these demonstrate that MaxCyte electroporation can be used to engineer difficult-to-transfect human immune cells for therapeutic applications. In particular, MaxCyte enabled both transient and stable expression of CARs or TCRs in T cells, NK cells and macrophages through the efficient delivery of various cargo, including mRNA, DNA or CRISPR RMPs. In addition, cells engineered using MaxCyte remained viable and functional both in vitro and in vivo.