Perry Rearick: Joining us now is Megan Embrey. She is a senior field application scientist with MaxCyte. Cell and gene therapies are revolutionizing treatments for cancer and other serious diseases, but immune cell engineering remains difficult. Due to the sensitivity and resistance of standard transfection, MaxCyte’s ExPERT electroporation platform offers a highly efficient, scalable solution for delivering mRNA, DNA and CRISPR Cas9 RNPs into hard-to-transfect cells.
Megan's presentation highlights optimized workflows that streamline cell engineering from discovery to clinical and commercial production. Welcome, Megan.
Megan Embrey: Thank you for having me, Perry. I'm excited to talk to everybody today. We do have a poll question out here at the start. We'd really like to know what considerations are most important to you in selecting your cell therapy manufacturing platform.
I'm excited to share with you all how MaxCyte electroporation can help you navigate challenging cell engineering workflows with MaxCyte’s scalable and proven electroporation platform. I want to start with a little bit of background because cell and gene therapies are the fastest growing and most promising disease treatment option for patients battling cancer, genetic disorders, and really any serious disease.
And while the design of your therapy is going to be unique, the goal is always going to be the same: to provide a transformative, often curative solution for these patients while addressing that root cause at the cellular or genetic level.
There’s a fast-growing interest in in vivo applications, where those cells are modified inside the body through viral and non-viral vectors. However, as we've seen most recently, there are still a lot of hurdles associated with this method, including delivery, targeting and, sadly, as we saw in the last couple of weeks, safety. Ex vivo cell engineering, on the other hand, is a preferred strategy for many approved and late-stage therapies today. In this approach, cells are collected from the donor or patient, modified outside the body and then reintroduced after editing.
This process specifically allows for greater precision, better quality control and offers a wider range of more targeted cell populations. And while there's a range of ex vivo methods, including viral transduction and chemical transfection, often using a lipid carrier taken in by the cells slowly through endocytosis.
My focus today is going to be presenting on my personal favorite, electroporation. This method uses quick pulses of electricity to open up pores in the cell membrane, allowing your RNA, DNA, even RNP cargo to immediately enter the cell. This process is really fast, highly reproducible, and is especially powerful for those hard-to-transfect cells, including primary immune cells.
So please allow me to introduce you to MaxCyte. We are an electroporation company that is pioneering the field of non-viral ex vivo gene delivery. Our vision for the last 25 years has been to enable researchers to transform cells into therapies. At MaxCyte, we have a suite of instruments, all optimized to provide efficient delivery of your favorite molecule into your favorite cell. All of our instruments take up a very limited lab footprint, come with an integrated touchscreen and are operated using our intuitive electroporation software called the ExPERT Software. And while I think any one of these instruments could be a great addition to your lab, I briefly wanted to highlight our clinically validated GTx system.
MaxCyte’s GTx platform can rapidly transfect anywhere from 100,000 to 20 billion cells. This is a 21 CFR and cGMP-compliant instrument that also comes with access to our FDA master file, which then makes the IND filing process very painless. When you work with MaxCyte, you are actually working with a proven technology, as we enabled the first FDA non-viral cell therapy with our GTx system in 2024.
And while today I'm going to focus on cell and gene therapy applications, it is worth noting that electroporation is a powerful tool that can also streamline your workloads for cell-based assay development and your biologics matching. Most importantly, regardless of the technique or application that you're working with, MaxCyte wants to help you maintain a high level of reproducibility at any scale in your development.
We achieve this by using our uniquely designed reaction chambers, known as processing assemblies. I hope you can appreciate from this image that we have an unbroken line of consumables that we can offer, covering the small research scale all the way to a clinically relevant cell number, and then beyond, for large-scale biologics manufacturing.
All of this also occurs in MaxCyte’s universal electroporation buffer, so that's just one buffer, which helps keep your transfection process very simple. At MaxCyte, we really pride ourselves on providing a product that scales with you and doesn't require a lot of complicated re-optimizations as you scale up your process.
These conditions that we're optimizing at the small scale can be copied and pasted as you scale up to that large-scale equipment workflow. MaxCyte’s Flow Electroporation technology can integrate really quickly into a closed cGMP therapy workflow. As we've heard from some other presenters today, there are a lot of applications upstream and downstream of electroporation, and we are agnostic to those technologies and just slide nicely into all those cell therapy workflows.
Again, proof is in the pudding. MaxCyte enables a host of cell therapy clinical trials. Here we have a nice bullseye chart that shows both phase 1 and 2 clinical trials as well as pivotal. And as I mentioned earlier, the proven MaxCyte transfection enabling the commercial launch of Casgevy for sickle cell and beta cell thalassemia in 2024.
So whether you're looking to do allogeneic or autologous workflow flows, T cells, HSCs, MaxCyte is going to be a really good option for you.
Going forward, I want to present a few case studies that demonstrate MaxCyte’s capabilities for non-viral cell therapy manufacturing. Today I'm going to focus primarily on CRISPR-Cas9 workflows.
I want to share that MaxCyte is a flexible platform that can be paired with a wide range of cell engineering strategies. This can include base and prime editing. We also have success with clients using transposase workflows. And again, while we are focusing on non-viral, we do have many clients using us in a hybrid approach, so doing that CRISPR knockout using MaxCyte and then doing that CAR T knockin using a lentivirus or other viral cargo.
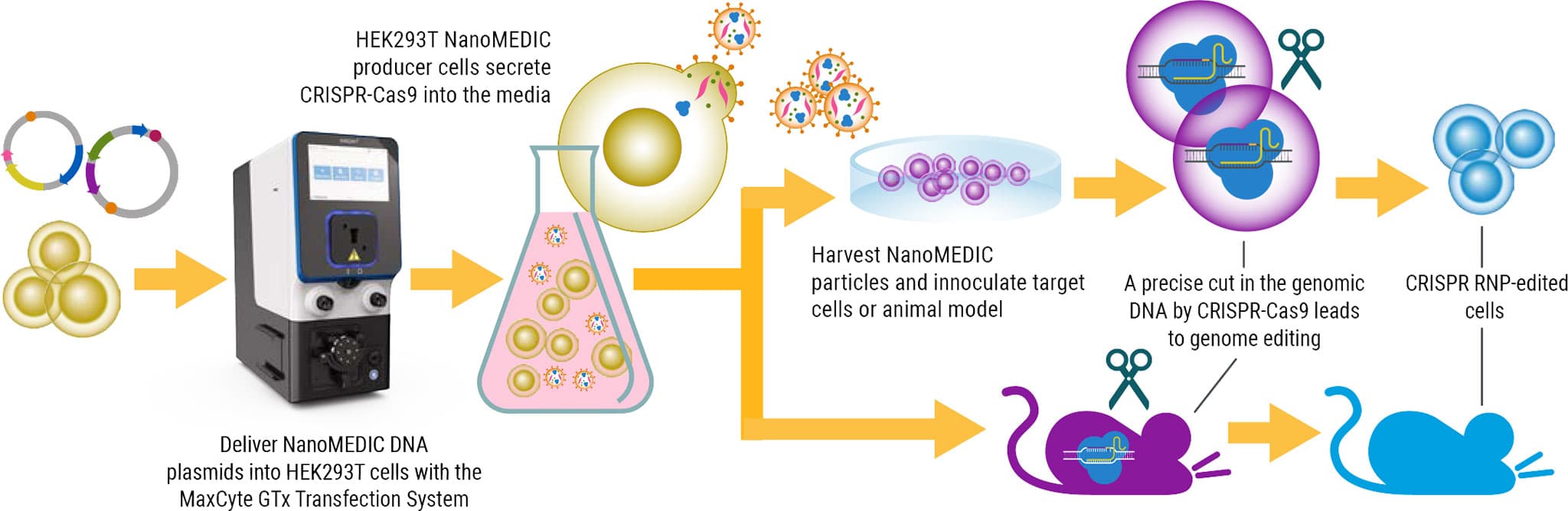
As many of you know, human-induced pluripotent stem cells or iPSCs play a really important role in disease modeling, drug screening and have a tremendous potential for regenerative medicine. So this story comes to us from the Hotta lab at CiRA, Kyoto University. And the goal of this group was to demonstrate and provide patient-derived iPSC cells that were engineered using CRISPR Cas9 for a range of diseases.
Now, when we first interacted with this customer, they were using another technology, so we really had to prove to them that MaxCyte was a powerful – and the correct – choice for their lab. We started with a basic experiment showing them the robustness of our instrument. In this experiment, we provided three different iPSC-specific energies for the transfection of an mRNA GFP-positive control.
As you can see, in all three cases, we saw over 100% transfection efficiency, all while titrating in a greater level of mRNA expression due to increased loading efficiency. They then moved on to the disease of interest. Their first disease they wanted to focus on was Duchenne muscular dystrophy, DMD, which is a devastating genetic disease that causes mutations in the DMD gene, leading to the absence of a functional protein for muscular function.
In this workflow, the Hotta lab wanted to use CRISPR homology-directed repair to introduce a reframing strategy that would result in a corrected healthy sequence for the gene of interest and introduce a restriction enzyme site for downstream analysis. Looking at the results of this CRISPR workflow with their original optimized electroporation compared to MaxCyte transfection, we can see not only a greater knockin efficiency, but I hope that you can really appreciate that reproducibility that we see with MaxCyte.
Interestingly on the right, I want to highlight that we can titrate in that knockin efficiency by varying the amount of ssODN that we use, achieving over 60% knockin with as little as five micrograms of ssODN for that same experiment.
We then looked at relative cell number as an indicator of cell viability. With MaxCyte electroporation alone, we see that there's a relatively low amount of cell loss, while the other electroporation system loses over 50% of their cells – and that's just with exposure to electroporation. Once we introduced RNP and ssODN, we do see some cell loss with both systems due to iPSC cells’ sensitivity to these cargoes, but again, the loss with MaxCyte is significantly less severe than with the other electroporation process.
From this first case study, I hope that you can really see where MaxCyte not only provides you with a consistent knockin efficiency but also a better viable product.
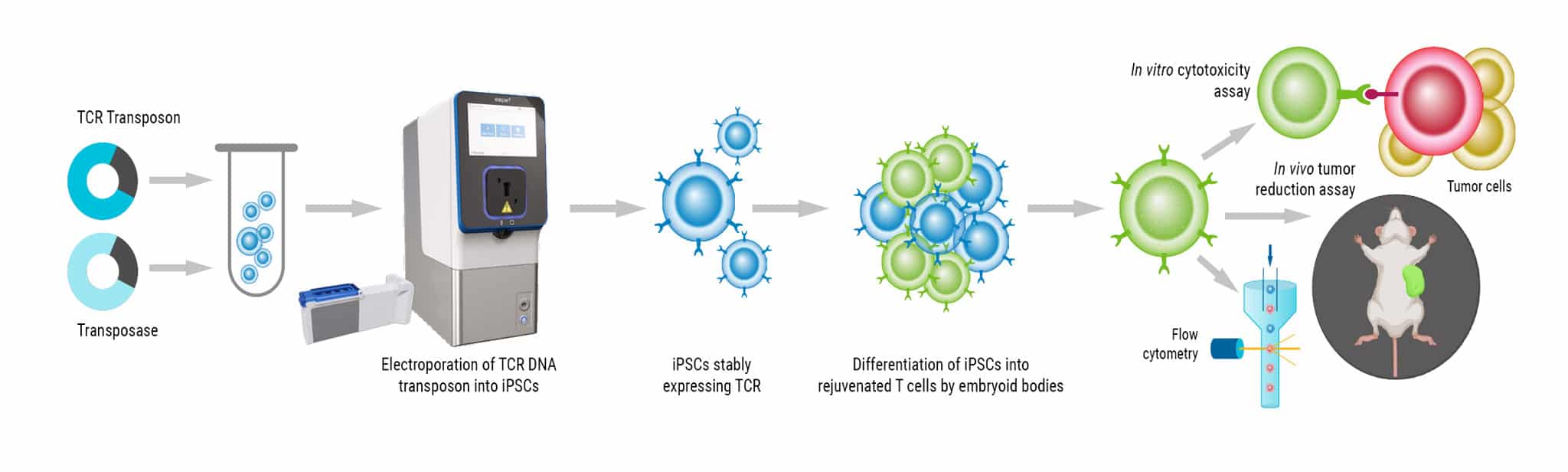
In our next case study, I wanted to highlight a program out of Spain where a group was going to develop a highly efficient CAR T cell treatment for lymphoma.
This group really wanted to move away from viral workflows to a more non-viral workflow to reduce complexity and cost of their therapy. And here, importantly, they'll be delivering mRNA and DNA for the Sleeping Beauty transposon system.
MaxCyte electroporation enabled a highly efficient transposition in both healthy and patient cells, which I think is a key feature to take away from this presentation. And not only were these cells highly functional, but they also expanded with a very rapid rate. This system was safe, as we saw the loss of transposase protein and unintegrated minicircle after seven and to 15 days, respectively.
Since your therapy is going to be on a global scale, it's really important to see how your product can be actually manufactured at different sites. And that's exactly what this researcher did. At two different manufacturing sites, they were able to reproduce that product with a high level of accuracy.
To close out my presentation here, I want to have you imagine working with a product that really strengthens the value of your pipeline, not only to your patients, but to anyone investing in your company. When you work with MaxCyte, you're working with a cGMP compliant platform that has a strong scientific support system. When you work with MaxCyte, you get a Megan. I really enjoy saying that. We can help you quickly have a commercial-ready process. And the support doesn't just stop with IND filing – this is a lifetime support process, supporting your efforts both domestically and globally.
To close out, we just scratched the surface on what MaxCyte can do. So I really encourage you to check out our website and our great social media team that’s trying to always present on the most interesting pieces of science to help you grow throughout your research. And thank you.
Perry Rearick: Thank you, Megan. Great job. We have some questions that are rolling in. We have a couple minutes.
Have you done any side-by-side tests, comparisons to other systems that are available in this market? And what kind of conclusions have you drawn, without poking any competitors in the eye? We don't want to do that, but what have you learned?
Megan Embrey: Of course. I would point back to that earlier study with the iPSCs. Obviously, we didn't want to call out anyone specifically, but we find with MaxCyte, you really get to balance a high viability with a high transfection efficiency, so you're not just getting one or the other; you're getting both.
And then importantly, I think this is a pain point for a lot of other systems. We have a scalable, seamless nature, meaning that whatever you're doing at that small scale really is reproducible at that large scale without any re-optimization at each level. And then again, I'm proud of it. You get that unparalleled scientific support.
Perry Rearick: Okay. We'll move on to the next question, which is: Is there a cargo limit on how much can be transfected using MaxCyte?
Megan Embrey: The limit really depends on your cell type, and what you're trying to put in. There are challenges with larger cargoes, but that's really, again, where MaxCyte can shine because we can provide you that optimization and hold your hand, so you don't have to suffer in silence. We really want to see you excel quickly, so we can help you optimize quickly with those challenging workflows.
Perry Rearick: And can cells be cryopreserved after transfection?
Megan Embrey: Absolutely. I think that's an important part of this cell therapy workflow, right? You need to be able to dose these patients. MaxCyte electroporation can absolutely be paired with cryopreservation as early as one hour after transfection.
Perry Rearick: Great case studies. Those are always helpful. And you highlighted a CRISPR application. Can MaxCyte transfection be used with prime and base editing?
Megan Embrey: Yes, there's only so much we can talk about in 15 minutes, but we have a lot of great case studies on our website. I want to highlight that we actually are working with some of the leaders in prime and base editing. Please go check out our website for links to papers and application notes on those specific editing applications.
Perry Rearick: Well, Megan, thank you for being with us today. Megan Embry from MaxCyte. Thank you very much.