Optimizing VLP Manufacturing for Gene Editing by GMP-Compliant Flow Electroporation®
American Society of Gene and Cell Therapy Annual Meeting
New Orleans, Louisiana, USA
May 15, 2025
MaxCyte researcher Isabel Daher presents on optimizing VLP manufacturing for high yields in HEK cells
Abstract
Virus-like particles (VLPs) are non-infectious, virally derived nanoparticles that lack a viral genome. Upon packaging a CRISPR ribonucleoprotein complex (RNP), VLPs can target and transiently deliver cargo for genome editing in vivo to specific cell and tissue types. They are safer than traditional viral vectors as they are incapable of genomic integration. Scaling up VLP production can be cumbersome and time-consuming, requiring several rounds of optimization to transition from research to clinical scale. Additionally, consistency and reproducibility between batches is a major concern when relying on chemical methods of VLP production.
Here, we utilized the MaxCyte ExPERT GTx®, a GMP-compliant electroporation instrument, to manufacture clinical-scale VLPs in adherent and suspension HEK cells, packaged with CRISPR-Cas9 or adenine base editor RNPs, for genome editing in primary human cells.
We found that electroporation consistently produced high yields of functional VLPs with an optimized electroporation workflow. We also demonstrated effective gene editing at several therapeutically relevant loci in primary hematopoietic cells, such as B2M and PD-1. Furthermore, the production of VLPs using electroporation exhibited favorable production kinetics compared to other transfection methods, enabling a one-day manufacturing process. Finally, we highlight the scalability of VLP production across a 400-fold volume range with minimal re-optimization, transfecting over one billion cells per production.
In summary, our results show that MaxCyte’s Flow Electroporation® technology is a viable means for consistent, efficient and scalable manufacturing of VLPs for gene editing applications and has high promise to address the needs of future clinical and commercial manufacturing.
Key takeaways
- The MaxCyte ExPERT Flow Electroporation platform enables VLP production in both adherent and suspension HEK293 cells.
- Incorporation of a variety of RNPs into VLPs via electroporation compares favorably to chemical transfection.
- VLPs produced via electroporation are potent, directing high-efficiency gene editing at low doses, and can be achieved within a one-day production timeline.
- VLP production is scalable, from 5 million to 10 billion cells, without the need for additional process development, maintaining a titer comparable to small scale.
Watch the presentation on process optimization for high-yield VLP manufacturing

View more details and illustrations explaining how MaxCyte's electroporation platform enables scalable VLP production in HEK293 cells
Have more questions?
Send your question to one of our cell engineering experts.
Presenter

Related resources
Poster presentation transcript
Isabel Daher: Hello, my name is Isabel Daher, and I'm excited to share with you MaxCyte’s efficient, large-scale VLP manufacturing for gene editing by our GMP-compliant Flow Electroporation® platform.
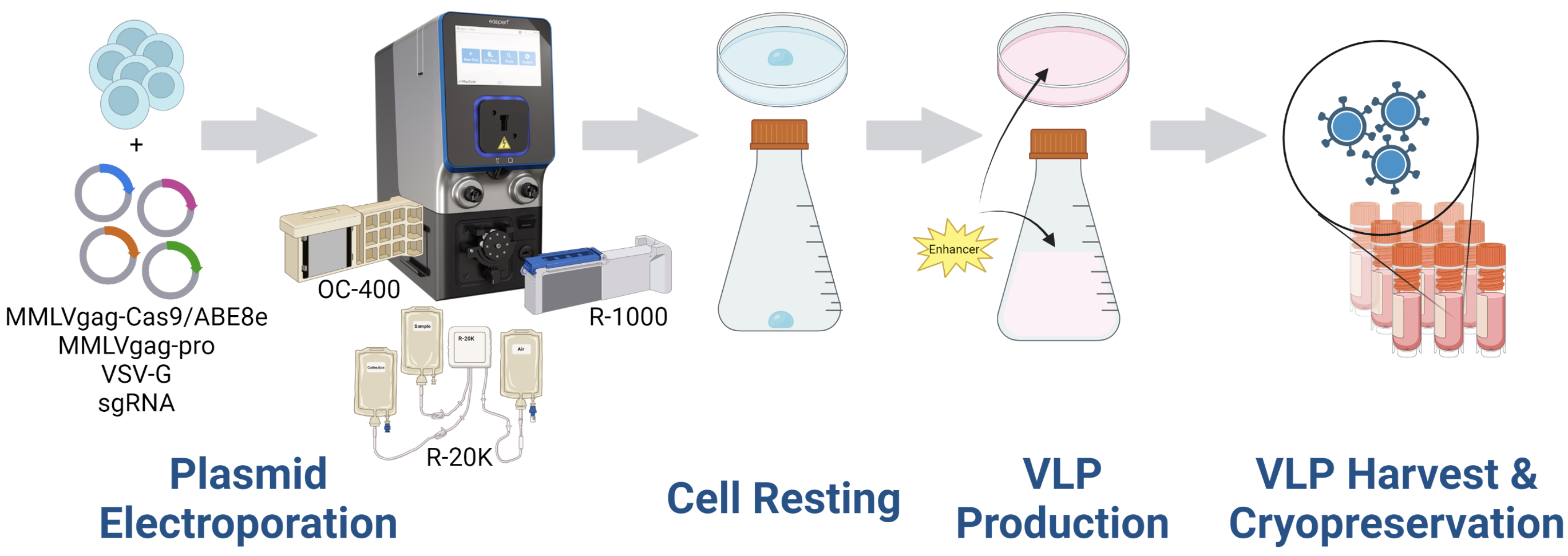
Our workflow is detailed here, where we begin by preparing for electroporation by mixing producer cells with a fixed-ratio plasmid DNA cocktail. The suspension is then loaded into the processing assembly, or PA, and then inserted into the instrument for electroporation with a preloaded and optimized protocol. The electroporated cells are then transferred into the final culture vessel for a short rest period. And then production media and an enhancer is added for the remainder of the production.
After one day, VLPs are harvested from the supernatant, purified and concentrated, and then cryopreserved. To assess our yields, concentrated VLPs are serially diluted and added to the target cells. After three to five days, cells are harvested for downstream analytics, such as amplicon sequencing or flow cytometry.
In adherent HEK293 cells, our platform can scale VLP production from five to 40 million cells in the R-50, OC-100 or OC-400 PA without any significant losses in editing capacity or titer. Here, we compare electroporation [EP] to chemical transfection. EP cannot only enable a shorter production timeline where VLPs are harvested within one day of electroporation, but also they exhibit a higher physical titer when produced via EP as compared to other methods.
When preparing for clinical or commercial manufacturing, suspension HEK293 become the producer cell of choice due to ease of handling and scalability. Here, we show that electroporation facilitates scalable and high-titer VLP production from 40 to 240 million suspension HEK cells. And again, we compared electroporation to an alternative transfection method and find that VLPs with high editing capacity can be harvested within one or two days of electroporation.
Interestingly, when normalized to p30, electroporation yields four to five times more Cas9 incorporation than chemical transfection. Thus far, all gene editing presented here has been performed in HEK293T at the HEK3 locus as a model system. But when reprioritizing efforts to relevant loci in primary cells, efficiency may decrease.
To assess editing and therapeutically relevant loci, we first edited the B2M locus in HEK293T cells and confirmed high indel formation. We then correlated indel formation with B2M knockout, measured via flow cytometry, and saw a strong association. We then attempted to edit B2M in activated T cells and achieved over 70% B2M negative cells.
Previously, I shared that electroporation could enable high Cas9 incorporation into the VLPs. Now we've attempted to package adenine base editors in replacement of the wild type Cas9 and show that at the HEK3 and B2M loci VLPs are capable of a high degree of deamination of bases within the optimal editing window.
This base editing is translated well by the associated knockout of B2M in HEK293T and activated T cells measured via flow cytometry. While most process optimizations or screenings may take place at small scale, once a candidate VLP is identified, one is ready to manufacture for large-scale studies.
Here, we show a comparison of both small- and large-scale EP, coupled with small- or large-scale VLP production culture. Without the need to reoptimize electroporation or culture conditions, we can scale VLP production up to 15 milliliters with the CL-2 processing assembly, which is capable of transfecting between one and 10 billion cells, and with large-scale VLP production, there is no loss in editing capacity or p30 titer.
By now, I hope to have shown you that our platform enables a VLP production in both adherent and suspension HEK293 cells and that different gene editor RNPs can be incorporated into capsids with high efficiency. All VLPs produced with electroporation exhibited high editing capacity, and this can be achieved within one day of electroporation, minimizing production timelines.
And finally, our process is scalable from five million to 10 billion cells, a 2,000-fold increase in cell number, and requires no additional optimizations or modifications to the workflow to maintain high productivity. Thank you for attending, and we look forward to working with you for your VLP production needs.