The American Society of Gene and Cell Therapy’s (ASGCT) annual meeting is the premier event for professionals in gene and cell therapy. The meeting is the best place for people in the field to learn from the latest scientific research, stay up to date on new technologies and make career-advancing connections with peers. Originally designed as a venue for academic researchers to share their work, the annual meeting has grown to serve a wide community including clinicians, bio-industry development, regulatory agencies, equipment manufacturers and patient advocates.
MaxCyte showcased our cGMP-compliant nonviral cell engineering platform and how we can accelerate therapeutic development from concept to clinical utility.
Featured Presentation
Overcoming Challenges in Translating Research Development to a Scalable Clinical Manufacturing Process
Thursday, May 9, 2024, from 8:30 to 9:30 a.m. ET in Room 309-310
From bench to bedside. We will explore the complexities of translating research into scalable manufacturing processes for cell and gene therapies. Executives from the industry will share their insights and innovative strategies for navigating obstacles, the importance of collaboration and technological innovation as well as regulatory compliance to help advance transformative therapies. Join us as we delve into the critical intersection of research development and clinical manufacturing to accelerate the development of life-saving treatments. This session will include a roundtable discussion followed by a presentation from KSQ Therapeutics on developing a scalable cGMP process for their engineered tumor-infiltrating lymphocyte (eTIL™) cell therapy.
Speakers during our presentation




Networking Event
Wrap the day with fellow researchers while meeting the MaxCyte team
Wednesday, May 8, 2024, from 7:00 to 9:30 p.m. ET
Blackwall Hitch at 700 E Pratt St, Baltimore, MD 21202
We are inviting all attendees of the ASGCT annual meeting to an evening of networking. Join us at this exclusive event where you will have the opportunity to meet the MaxCyte team. Enjoy complimentary hors d’oeuvres and beverages while mingling with fellow scientists and researchers.
Registration for this event has now closed.

Oral presentation
Non-Viral Engineering of Primary Human Macrophages Using a cGMP-Compliant Electroporation Platform
Presented by Ashley Strickland-Dietz, PhD, Immunology Scientist I at MaxCyte
Friday, May 10, 2024, in Ballroom 2 from 5:15 to 5:30 p.m. ET
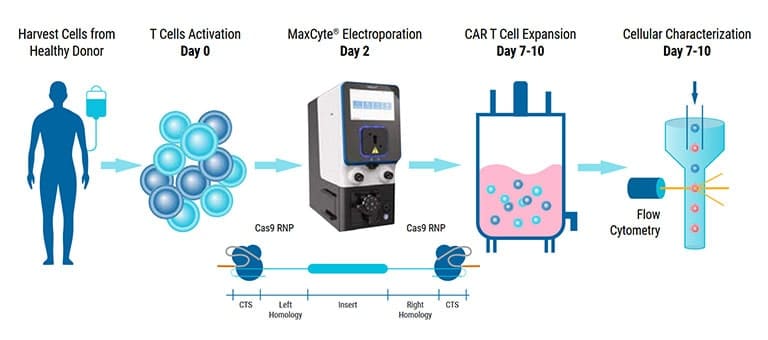
Macrophages are highly plastic innate immune cells that can exhibit pro- or anti-inflammatory effector functions depending on their activation state. For this reason, macrophages can be used for various therapeutic applications ranging from cancer treatment to regenerative medicine. To engineer macrophages for these purposes, biomolecules or other genome editing tools must be delivered into these cells. Electroporation, also known as electropermeabilization, is an effective nonviral transfection method that utilizes an electric field to facilitate the passage of molecules across biological membranes. Here, it is demonstrated that highly efficient delivery of mRNA, DNA and CRISPR ribonucleoproteins (RNPs) into primary human macrophages can be achieved using the MaxCyte ExPERT GTx system - a cGMP-compliant electroporation platform. In fact, use of the ExPERT GTx to transfect macrophages with mRNA or SIRPα-targeting CRISPR RNPs resulted in efficiencies greater than 90% with minimal impact on cell viability. This system also enabled delivery of DNA, ranging in size from 2.5-kb to 9.2-kb, into macrophages with transfection efficiencies between 60% and 90%. These efficiencies were inversely related to plasmid size and were accompanied by a dose-dependent decrease in cell viability. Despite this, electroporation was determined not to directly impair migration, activation/polarization, or phagocytosis in viable macrophages. For further proof-of-concept, the ExPERT GTx was also used to generate clinically relevant chimeric antigen receptor (CAR)-expressing macrophages targeting the tumor antigen CD19. These CAR macrophages were found to be functional and highly effective at eradicating CD19+ target cells. Together, these demonstrate that the MaxCyte ExPERT GTx electroporation platform can be used to engineer macrophages for use in cell therapy applications.
Poster presentations
Non-Viral Cell Engineering of Peripheral Blood Natural Killer Cells Using a GMP-Compliant, Scalable Electroporation Platform

Poster 861 presented by Lauren Unsworth, Research Associate II at MaxCyte
Wednesday, May 8, 2024, in the Exhibit Hall from 12:00 to 7:00 p.m. ET
Natural killer (NK) cells are an integral part of the innate immune system as they recognize an abundance of surface receptors on target cells without the need for antigen specificity and recruit and activate other immune cells by secreting a myriad of cytokines. The hallmark of NK cells is their cytotoxic function which enables them to recognize and attack tumor or infected cells by releasing granules that induce apoptosis of target cells. Electroporation (EP) is a nonviral method for cellular engineering that has been used with virtually all cell types and has been specifically shown to be an effective cell engineering method for peripheral blood NK cells. Advantages of electroporation include highly efficient transfection with a variety of molecules (e.g., DNA, RNA, and ribonucleoprotein (RNP) complexes), regulatory acceptance, and cGMP compatibility. In this study, we optimized conditions for electroporating primary NK cells with the GMP-compliant MaxCyte ExPERT GTx electroporation platform, with specific focus on maximizing cell viability, cell recovery, transfection efficiency, cell expansion and functionality. Previous studies showed that expansion of NK cells before electroporation was necessary for effective transfection, and therefore larger scale platforms were required, leading to the use of more costly reagents. Here, we showed that the MaxCyte ExPERT GTx could genetically modify naive or expanded primary NK cells using mRNA, RNP complexes or plasmid DNA at both a small (1x106 cells in 25µl) and larger scale (1x108 cells in 5ml). We observed over 90% gene expression and viability following mRNA electroporation and over 60% expression and viability after minicircle DNA (mcDNA) loading at both small and larger scales. We also evaluated the effect of commercially available DNA sensing pathway inhibitors to determine if transfection efficiency and cell viability could be increased when loading small and large plasmid DNA. Furthermore, we knocked-out the NKG2A inhibitory receptor at different timepoints during expansion by loading a sgRNA-Cas9 RNP complex and looked at post-electroporation effects. With our optimized transfection conditions specific for NK cells, we determined that electroporation had minimal impact on cell viability, cell expansion, surface marker expression and killing capacity.
Efficient Scalable Manufacturing of Virus-Like Particles for the Delivery of CRISPR-Cas9 Ribonucleoproteins Using a cGMP-Compliant Electroporation Platform

Poster 1733 presented by Isabel Daher, Research Associate at MaxCyte
Friday, May 10, 2024, in the Exhibit Hall from 12:00 to 7:00 p.m. ET
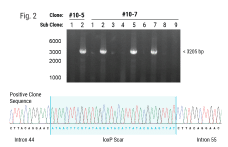
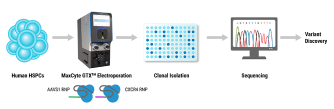
Genome editing tools such as CRISPR-Cas9 nucleases, base editors, and prime editors hold tremendous promise for treating human diseases by being able to specifically modify a targeted region of the DNA genome. However, there are limited in vivo delivery options that are safe, efficient and transient. Moreover, viral vectors such as AAV are hampered by limited cargo size capacity. Virus-like particles (VLPs) offer a solution to these problems. VLPs are derived from retroviral structural proteins, which can be engineered to specifically package a cargo of interest. They are safer than traditional viral vectors because they lack a viral genome but can utilize the traditional virus delivery machinery to target and enter cells. Recently, VLPs have been reported to efficiently package base editors and prime editors for delivery into mice at therapeutically relevant levels. To realize the potential of VLPs for in vivo delivery, alternative methods to scale up their manufacturing will be critical for future clinical applications. Here, we utilized the MaxCyte ExPERT GTx, a cGMP-compliant electroporation instrument, to manufacture VLPs— packaged with CRISPR-Cas9 RNPs for genome editing in target cells — in adherent and suspension HEK293 cells. We found that electroporation consistently produced significantly higher yields of functional VLPs compared to a commercially available transfection reagent, with up to a 20-fold improvement when using an optimized electroporation protocol. Furthermore, the production of VLPs using electroporation exhibited favorable production kinetics compared to other transfection methods, enabling a shorter VLP manufacturing process. Finally, we demonstrated the scalability of VLP production across a 15-fold volume range with minimal re-optimization. In summary, our results show that electroporation is a viable means for consistent, efficient and scalable manufacturing of VLPs for gene-editing applications and has high promise to address needs for future clinical and commercial VLP manufacturing.