The American Society of Gene and Cell Therapy’s (ASGCT) annual meeting is the premier event for innovation in gene and cell therapy—bringing together researchers, clinicians, industry leaders and advocates to share breakthroughs, explore new technologies, and spark meaningful collaboration.
Come visit MaxCyte® at booth #1545 to explore our cGMP-compliant, non-viral cell engineering platform designed to streamline and accelerate the development of cell and gene therapies from concept to clinic. Discover how our technology empowers seamless, scalable and high-performance cell modification to support your therapeutic pipeline
Featured presentation
Reducing risk in gene editing programs through robust off-target safety evaluation
Thursday, May 15, 2025, from 12:15 to 1:15 p.m. CT in Room 271-273
Presenters


Poster presentations
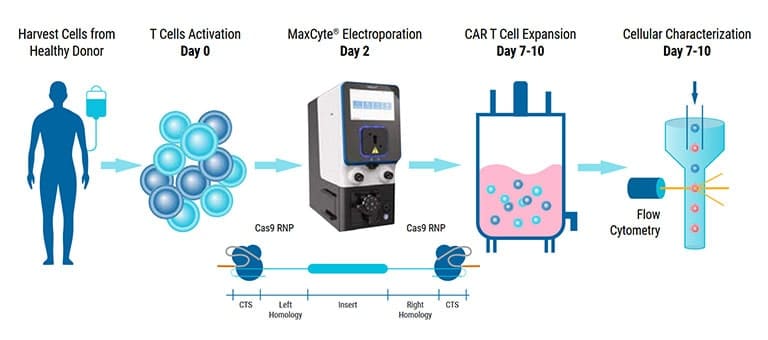
Optimizing CRISPR/Cas-mediated CAR knockin in primary human T cells using MaxCyte electroporation

Poster presented by Ashley Strickland-Dietz, Immunology Scientist at MaxCyte
Thursday, May 15, 2025, in Poster Hall 12 from 5:30 - 7:00 p.m. CT
In recent years, chimeric antigen receptor (CAR) T cells have emerged as a leading treatment for various hematological cancers. Despite demonstrating great promise, CAR T cell therapies are limited by several challenges, including concerns regarding efficacy, safety and manufacturability. To address these, groups have turned to non-viral engineering of CAR T cells using CRISPR gene editing. In addition to reduced immunogenicity and manufacturing costs, this technology enables precise knockin of tumor-targeting receptors and knockout of genes responsible for rejection, toxicity and immunosuppression. To CRISPR engineer CAR T cells, Cas nucleases, guide RNA (gRNA) and homology-directed repair (HDR), template DNA must be efficiently delivered into T cells without jeopardizing their viability or functionality. With this in mind, we sought to generate optimized workflows that enable CRISPR-mediated gene editing in primary human T cells using MaxCyte electroporation. To this end, we first identified optimal electroporation protocols and concentrations of sgRNA, Cas9 and HDR template required to knock-in GFP at the TRAC locus. Next, we compared different repair template designs, including the addition of Cas9 targeting sequences (CTS). We also investigated multiple HDR template DNA formats, including linear double-stranded DNA, single-stranded DNA, conventional plasmids and Nanoplasmids™. In addition, we explored the use of small molecule enhancers and identified several that further improved editing efficiencies. These findings were then used to engineer CD19-targeting CAR T cells. With activated T cells from healthy donors, we could achieve CAR expression levels of greater than 70 percent. In addition to efficient and reproducible CAR knockin in T cells from multiple donors, cells engineered using this workflow were viable and retained the ability to expand and eradicate CD19-expressing target cells. Together, these demonstrate the capability of MaxCyte’s clinically validated ExPERT™ electroporation technology to enable non-viral engineering of CAR T cells using the CRISPR/Cas system.
Efficient, large-scale, virus-like particle manufacturing for gene editing by a GMP-compliant flow electroporation platform

Poster presented by Isabel Daher, Research Associate at MaxCyte
Thursday, May 15, 2025, in Poster Hall 12 from 5:30 to 7:00 p.m. CT
Virus-like particles (VLPs) are non-infectious, virally derived nanoparticles that lack a viral genome. Upon packaging a CRISPR ribonucleoprotein complex (RNP), VLPs can target and transiently deliver cargo for genome editing in vivo to specific cell and tissue types. They are safer than traditional viral vectors as they are incapable of genomic integration. Scaling up VLP production can be cumbersome and time-consuming, requiring several rounds of optimization to transition from research to clinical scale. Additionally, consistency and reproducibility between batches is a major concern when relying on chemical methods of VLP production. Here, we utilized the MaxCyte ExPERT GTx®, a GMP-compliant electroporation instrument, to manufacture clinical-scale VLPs, in adherent and suspension HEK cells, packaged with CRISPR Cas9 or adenine base editor RNPs for genome editing in primary human cells. We found that electroporation consistently produced high yields of functional VLPs with an optimized electroporation workflow. We also demonstrated effective gene editing at several therapeutically relevant loci in primary hematopoietic cells, such as B2M and PD-1. Furthermore, the production of VLPs using electroporation exhibited favorable production kinetics compared to other transfection methods, enabling a one-day manufacturing process. Finally, we highlight the scalability of VLP production across a 400-fold volume range with minimal re-optimization, transfecting over one billion cells per production. In summary, our results show that MaxCyte’s Flow Electroporation® technology is a viable means for consistent, efficient and scalable manufacturing of VLPs for gene editing applications and has high promise to address the needs of future clinical and commercial manufacturing.
Optimizing transposon-based gene delivery for cell therapy applications with the MaxCyte ExPERT scalable electroporation platform

Poster presented by Max Van Buskirk, Research Associate at MaxCyte
Tuesday, May 13, 2025, in Poster Hall 12 from 6:00 to 7:30 p.m. CT
In recent years, chimeric antigen receptor (CAR) T cells have emerged as a leading treatment for various hematological cancers. Despite demonstrating great promise, there are several challenges that limit CAR T cell therapies including concerns regarding efficacy, safety and manufacturability. To address these, groups have turned to non-viral engineering of CAR T cells using CRISPR gene editing. In addition to reduced immunogenicity and manufacturing costs, this technology enables precise knockin of tumor-targeting receptors and knockout of genes responsible for rejection, toxicity and immunosuppression. To CRISPR engineer CAR T cells, Cas nucleases, guide RNA (gRNA) and homology-directed repair (HDR), template DNA must be efficiently delivered into T cells without jeopardizing their viability or functionality. With this in mind, we sought to generate optimized workflows that enable CRISPR-mediated gene editing in primary human T cells using MaxCyte® electroporation. To this end, we first identified optimal electroporation protocols and concentrations of sgRNA, Cas9 and HDR template required to knock in GFP at the TRAC locus. Next, we compared different repair template designs, including the addition of Cas9 targeting sequences (CTS). We also investigated multiple HDR template DNA formats, including linear double-stranded DNA, single-stranded DNA, conventional plasmids and nanoplasmids. In addition, we explored the use of small molecule enhancers and identified several that further improved editing efficiencies. These findings were then used to engineer CD19-targeting CAR T cells. With activated T cells from healthy donors, we could achieve CAR expression levels of greater than 70%. In addition to efficient and reproducible CAR knockin in T cells from multiple donors, cells engineered using this workflow were viable and retained the ability to expand and eradicate CD19-expressing target cells. Together, these demonstrate the capability of MaxCyte’s clinically-validated ExPERT™ electroporation technology to enable non-viral engineering of CAR T cells using the CRISPR/Cas system.
A robust approach for CRISPR-Cas therapeutic guide design and selection leveraging variant-aware computational and biochemical approaches

Poster presented by Vijetha Vemulapalli, PhD, Vice President of Data Science at SeQure DX, A MaxCyte Company
Tuesday, May 13, 2025, in Poster Hall 12 from 6:00 to 7:30 p.m. CT
Guide RNA (gRNA) design for a CRISPR-Cas system routinely begins with computational tools to identify a small set of candidate guide sequences. While currently available computational tools that are designed for guide design take into account factors that impact on-target editing, they do not provide a comprehensive analysis of off-target risk or genetic variation present in human populations. Moreover, any off-target analysis is based on predictions from statistical models with no consideration for biological significance of putative off-target loci. These computational analyses are often followed by selection of a single guide based on experiments evaluating editing efficiency in a relevant model system without any biochemical assessment for off-target specificity. Motivated by experience in reducing off-target gene editing risk in therapeutic applications, we have developed a more effective approach.
Here, we present an integrated, multi-step approach that utilizes a combination of computational tools (Guide Designer, Guide Profiler™) and an in-vitro biochemical assay (Guide Select™) to perform variant and off-target aware guide design, profiling and selection.
The TRBC1 gene is a well-studied therapeutic target in immunotherapies. Using the Guide Designer software, we performed comprehensive design of candidate guides and profiled them based on several factors, such as location within the transcript and previously published metrics of on-target and off-target performance. For the top 25 gRNA sequences, the Guide Profiler software was used to perform a comprehensive search for on-target variation and candidate off-target sites across seven human superpopulations, annotation of putative off-target loci with biological features, and finally, rank-ordering of their off-target risk profile. For the top eight guide RNA sequences, this was then followed by empirically defining their biochemical editing specificity using the Guide Select assay. This assay is a multiplexed adaptation of the ONE-seq™ assay and is designed to biochemically identify off-target sequences with lower edit distance (less than five mismatches and indels) from the guide RNA sequence. Based on the assay readout, the guide RNA with lowest off-target risk is finally selected for downstream efficacy testing. Selection of the optimal candidate gRNA from this approach, and comparing to a previously published guide, we present data from off-target characterization using the GUIDE-seq™ and SAFER detection™ assays to underline the impact of this comprehensive guide RNA selection approach. In summary, improving the rigor around the design, profiling and selection of guides earlier in the discovery process reduces the overall risk of a therapeutic program, prioritizes safety and improves overall speed of a therapeutic program.
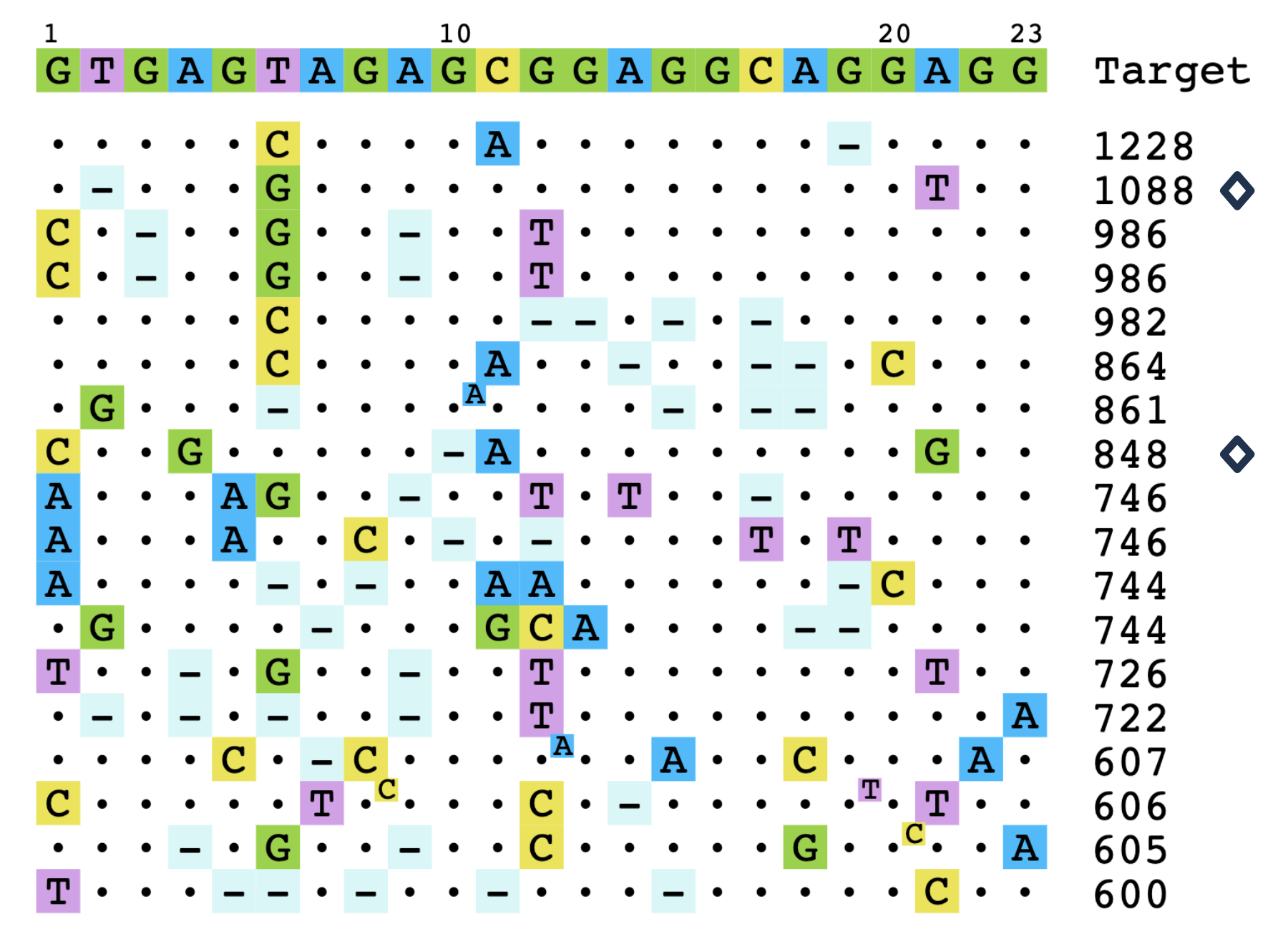
Sensitive rearrangement detection at CRISPR on- and off-target editing loci using SAFER Detection™

Poster presented by Doug Smith, Senior Vice President of Science and Innovation at SeQure DX, A MaxCyte Company Thursday, May 15, 2025, in Poster Hall 12 from 5:30 to 7:00 p.m. CT
Gene editing strategies that target multiple loci using engineered CRISPR Cas systems or other sequence-specific gene editing nucleases run a high risk of generating chromosomal rearrangements. Here, we applied Selective Amplification for Efficient Rearrangement Detection (SAFER detection) to detect intrachromosomal deletions and inversions as well as inter-chromosomal translocations involving loci for several therapeutic guide RNAs targeting CCR5, FXN, TRAC, TRBC1, TRBC2, B2M and PDCD1. The assay uses transposome-based tagmentation of genomic DNA, followed by selective amplification of rearranged molecules using a target-specific primer and locked nucleic acid blocker. The target specific primer is typically anchored near the on-target locus where double-strand breaks are intended to occur, but it can also be designed to bind near any known off-target locus. The assay can be performed in less than one week; it requires less genomic DNA than competitive methods and sensitively detects rearrangements involving the target locus in a genome-wide, unbiased way. Our analysis pipeline quantifies independent rearrangement events involving split reads with UMIs across multiple sample replicates, providing tabular and graphical summaries. Rearrangements with breakpoints in close proximity to regions of homologous sequence or off-target editing sites are classified as homology-mediated or off-target mediated, respectively.
For the CCR5 guide, we detected off-target and homology-mediated events at the CCR2 locus and off-target mediated translocations involving loci in chr13 19, and 20 that had been characterized previously by others using the same guide. A novel chromosomal translocation, mediated by off-target editing near oncogene PTP4A2 on chr1, was detected at an off-target site with three mismatches and one indel when aligned to the target. These sites were validated by PCR and dPCR. Editing with guides in the homologous regions of TRBC1 and TRBC2 exon 1 results in frequent deletions and inversions of the intervening region on chromosome 7. We also investigated a model system for treatment of Friedreich’s ataxia in which two guides at adjacent loci on chromosome 9 were used to edit a lymphoblastoid cell line from a patient with Friedreich’s ataxia carrying an expanded GAA repeat cluster at the Frataxin locus (Rocca et al., 2020). SAFER detection identified frequent deletions, as described in the initial study, but it also detected inversions of the GAA repeats between the two target loci.
The SAFER detection assay is complementary to nomination methods such as GUIDE-seq™ or ONE-seq™, as it provides a cost-effective approach to assess the risk of chromosomal rearrangements in therapeutic genome editing, and is especially valuable where multiplex editing strategies are employed. The method can also be adapted to identify viral or transposon integration sites.