Scientific Brief
MaxCyte® Enabled Development of Landing-Pad CHO Cell Line for Mammalian Display
Background
Since 1990, in vitro display technologies such as yeast and phage display, have been used to discover antibodies for research, diagnostic and therapeutic applications.1 More recently, mammalian display techniques have emerged, offering several advantages over earlier methods, including identifying candidates with reduced immunogenicity and improved stability and solubility.
In the mammalian display technique, candidate protein sequences are expressed in the chosen mammalian cells as membrane anchored proteins. Cells displaying the candidate proteins are screened and selected for desired protein properties such as antigen binding. Variations in the copy number and integration site of candidate sequences in mammalian display libraries created by viral transduction or standard transfection with random integration can complicate the interpretation of results. Expressing candidate sequences from one gene copy per cell integrated into a fixed genomic locus removes the need to normalize for variations in transcription.
Thus, to simplify mammalian display screening, a monoclonal high-expressing, single-site landing-pad cell line was developed to enable targeted, single-copy sequence integration by recombinase-mediated cassette exchange (RMCE) for mammalian display CHO cell library generation.2
Workflow overview for stable landing pad CHO cell line development
A
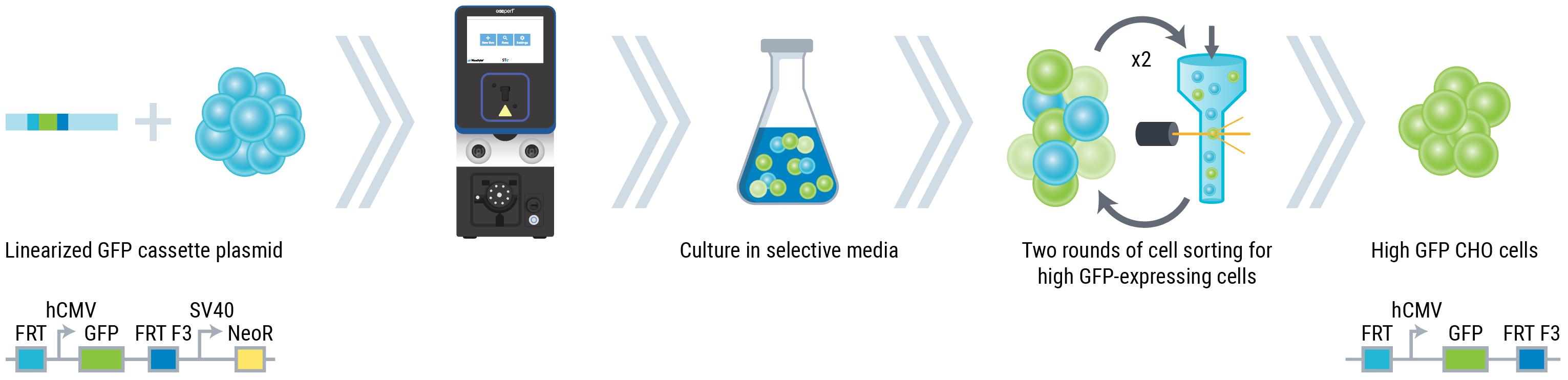
Figure 1: (A) CHO-S cells were transfected with a plasmid containing a GFP expression cassette flanked by Flp recombinase target sites and a neomycin resistance gene.
B
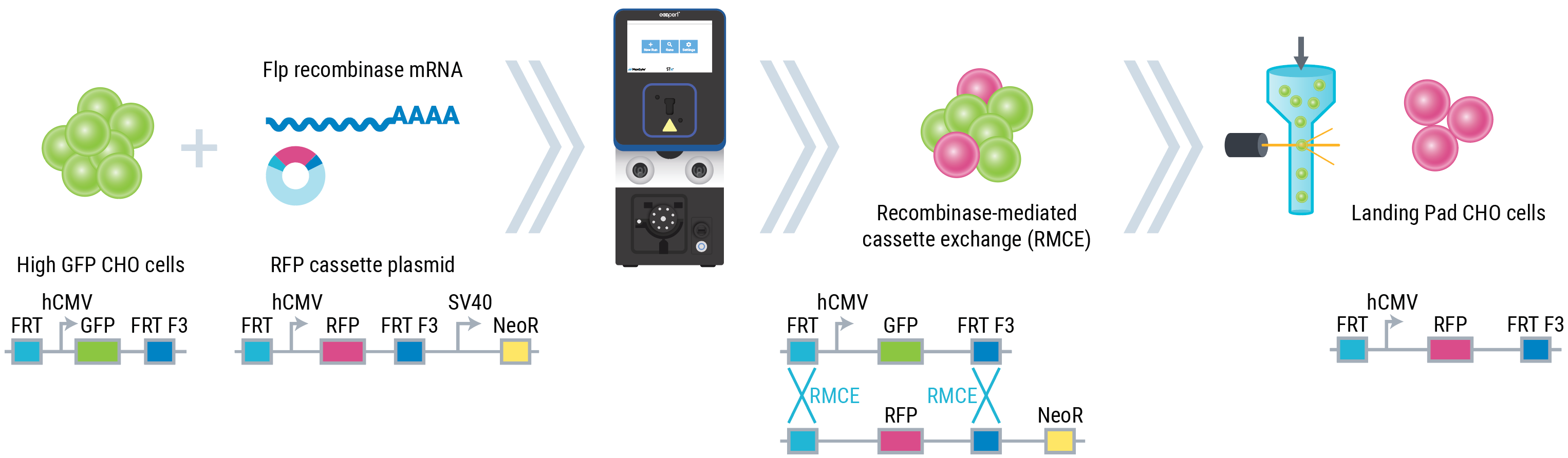
Figure 1: (B) High-GFP CHO cells were transfected with a linearized plasmid containing an RFP expression cassette flanked by Flp target sites and a Flp recombinase mRNA for recombinase-mediated cassette exchange.
Flow cytometric and fluorescence analysis
A
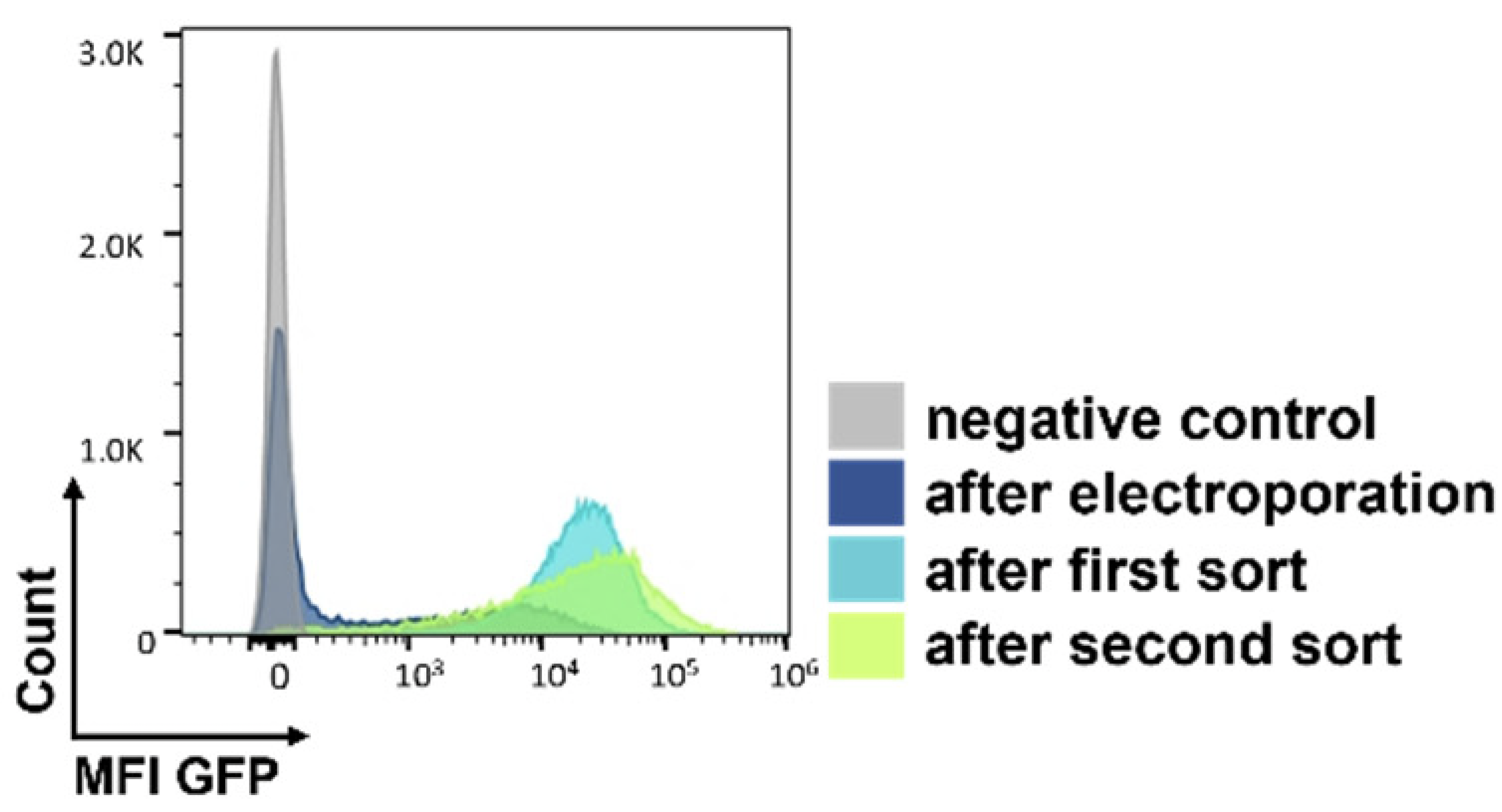
B
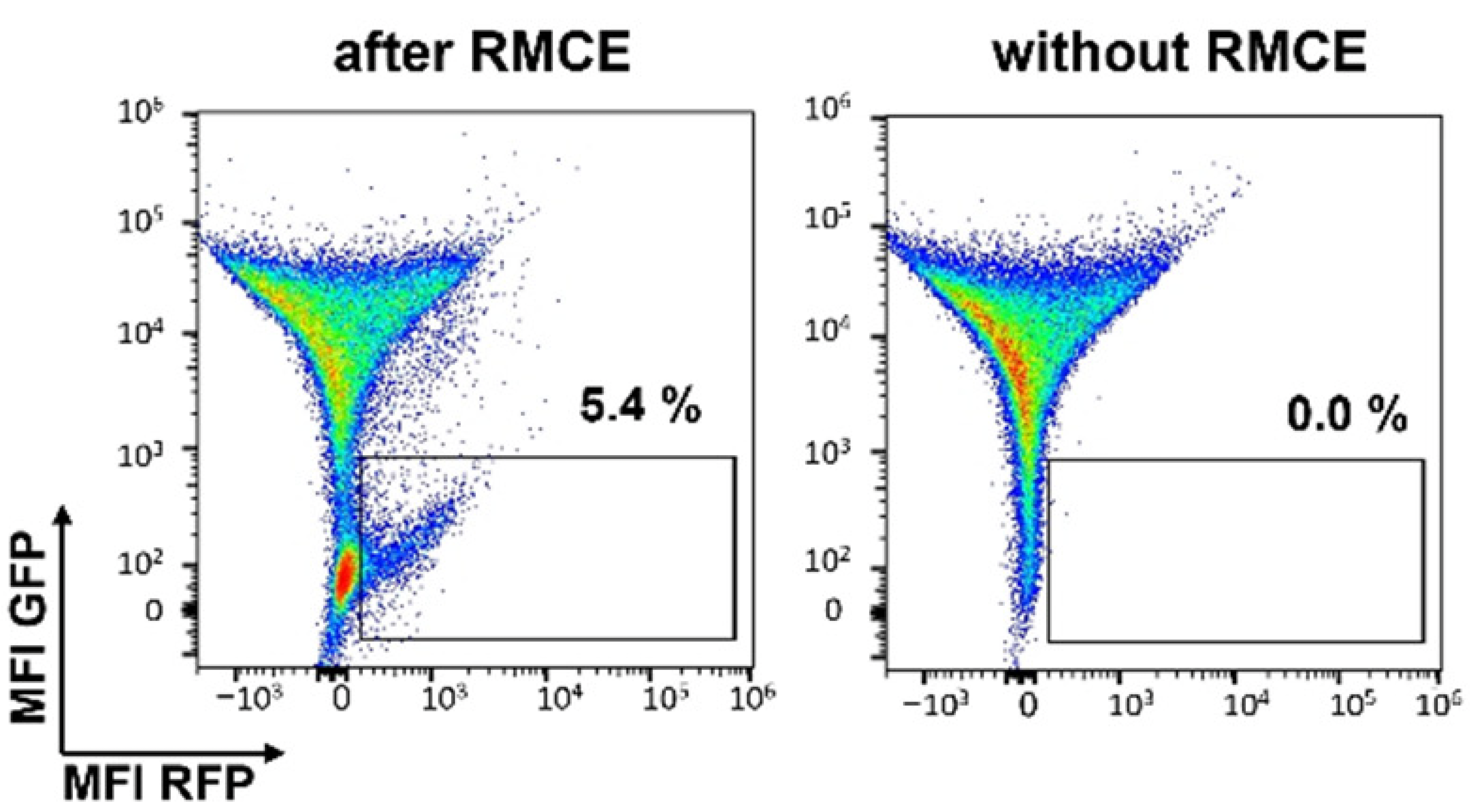
C
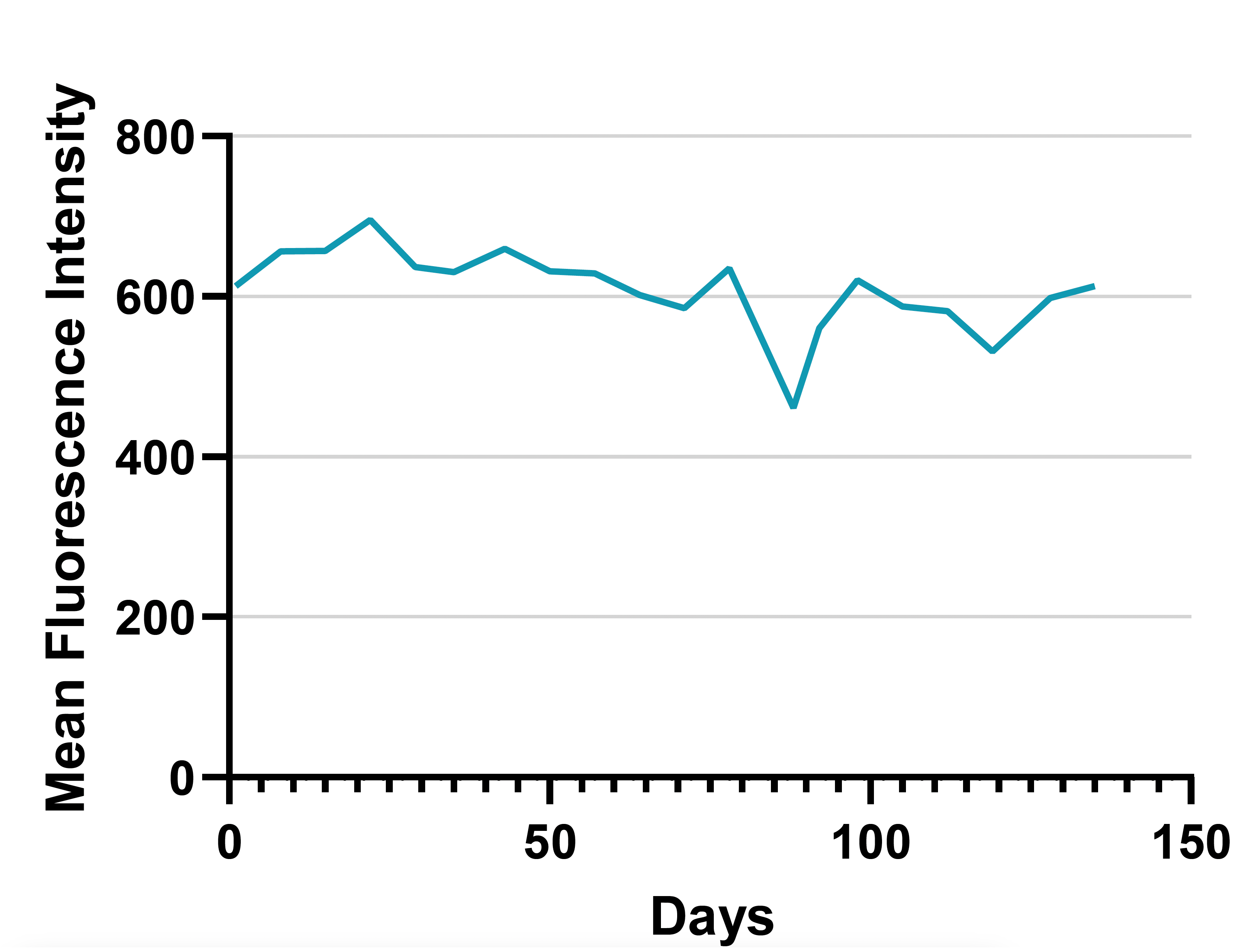
Figure 2: (A) Following two weeks of selection in media containing geneticin, the 2 percent of clones expressing the highest GFP levels were isolated by two rounds of cell sorting. (B) Following recombinase-mediated cassette exchange (RMCE), cells were analyzed by flow cytometry and sorted to isolate individual GFP negative/RFP positive clones. RMCE exchange efficiency of 5.4% compares favorably with other reports.1 (C) Individual clones were screened by targeted locus amplification to identify clones with single-copy integration. Clone RFP_A03 was chosen as the landing-pad-containing cell line (Landing Pad CHO), and the stability of RFP expression was confirmed over 135 days.
Summary
- MaxCyte electroporation enabled the development of a Flp landing-pad CHO cell line, Landing Pad RFP_A03 CHO, for simplified biologic drug discovery and development by mammalian display.
- MaxCyte electroporation delivered high-efficiency recombinase mediated cassette exchange for simple, rapid mammalian display cell library preparation.
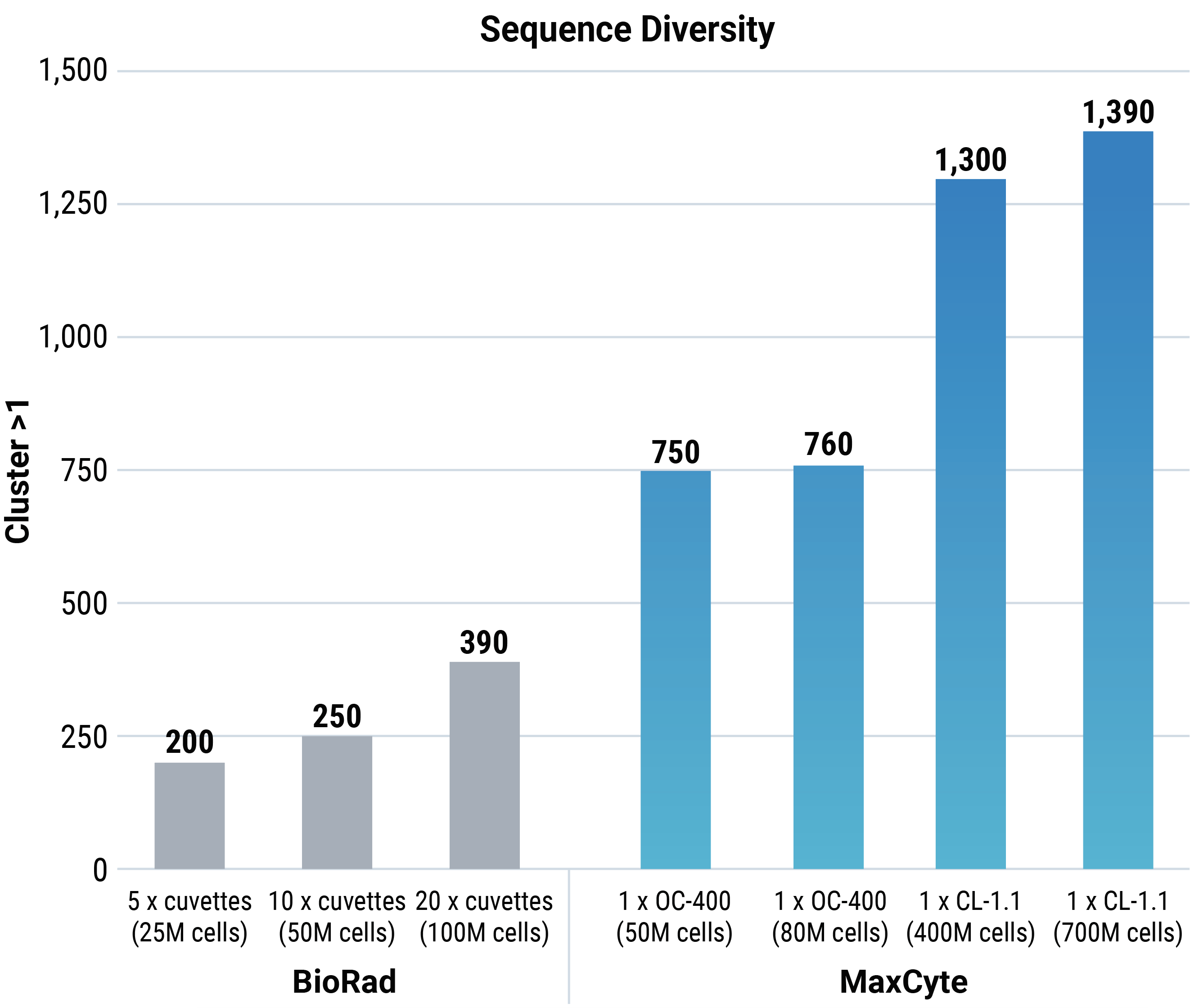
- MaxCyte’s Flow Electroporation® can transfect up to 2x1011 cells in a single reaction, theoretically enabling the generation of mammalian display cell libraries with up to 1x109 candidate sequences.
References
- Slavny P, Hegde M, Doerner A, et al. Advancements in mammalian display technology for therapeutic antibody development and beyond: current landscape, challenges, and future prospects. Front Immunol. 2024;15:1469329. Published 2024 Sep 24. doi:10.3389/fimmu.2024.1469329
- Dilchert J, Hofmann M, Unverdorben F, Kontermann R, Bunk S. Mammalian Display Platform for the Maturation of Bispecific TCR-Based Molecules. Antibodies (Basel). 2022;11(2):34. Published 2022 May 10. doi:10.3390/antib11020034