Scientific Brief
MaxCyte Enabled the Development and Rapid, Reproducible Manufacturing of TranspoCART Cells
Abstract
The development and commercialization of potentially life-saving CAR T cell therapies have been complicated by the cost and complexities associated with viral vector transduction. Viral vector production is expensive and time-consuming, the T cell transduction process may be problematic for sick patients, and there may be safety concerns about the unpredictability of the site of vector integration. MaxCyte’s clinically validated electroporation is more cost-effective, faster and can deliver multiple cargo types, enabling researchers to explore new cell engineering approaches and create better, more affordable therapies.
Here, researchers developed a non-viral CAR T process using MaxCyte electroporation to deliver CAR transposon minicircle DNA and Sleeping Beauty 100X transposase mRNA (SB100X) to autologous patient T cells. The manufacturing process was reproducible across two different sites and generated TranspoCART cells that prolonged survival in a mouse disease model.
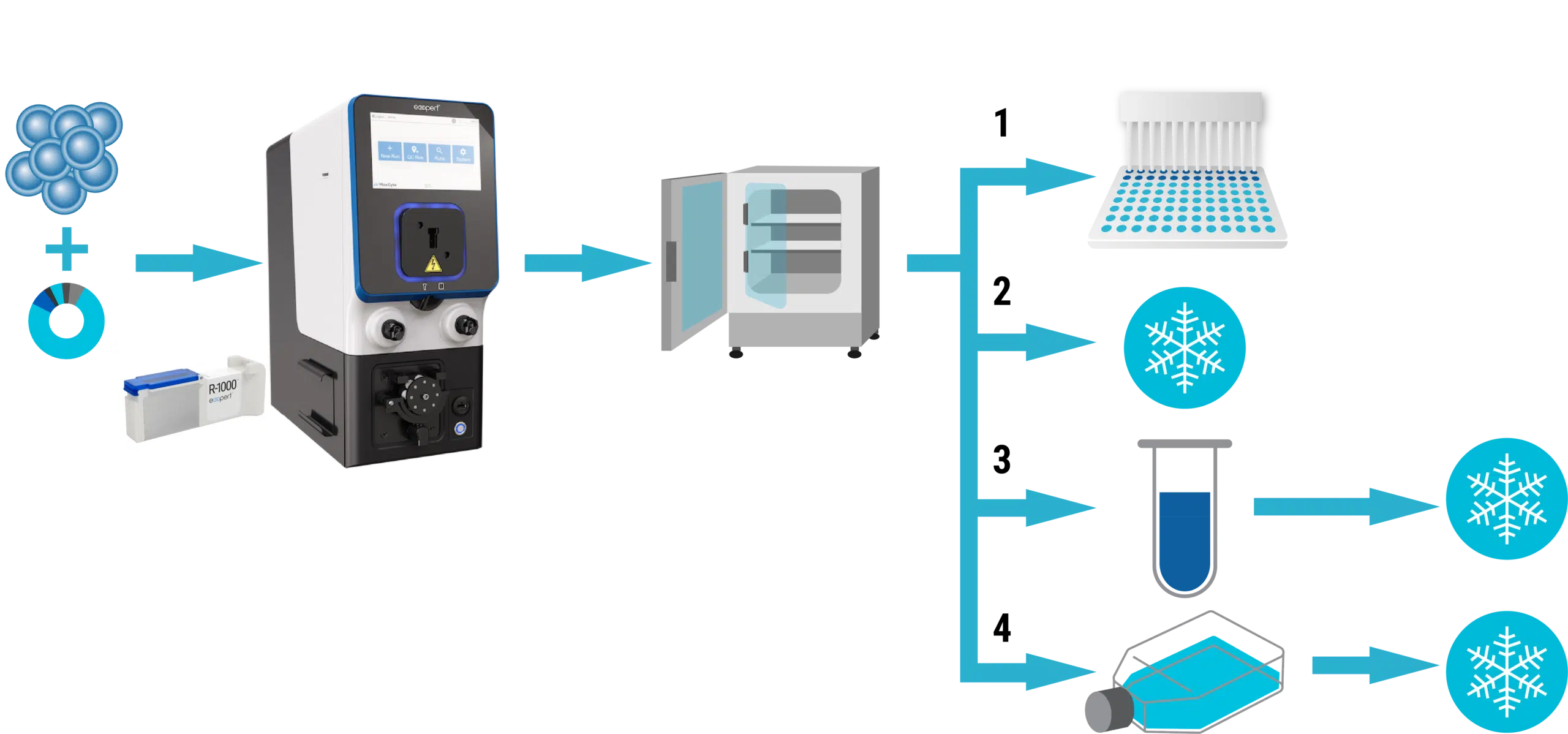
Workflow overview
The MaxCyte GTxTM was used to electroporate primary human T cells with minicircle DNA and transposase mRNA to generate TranspoCART cells, a new generation of CAR T cell therapies.
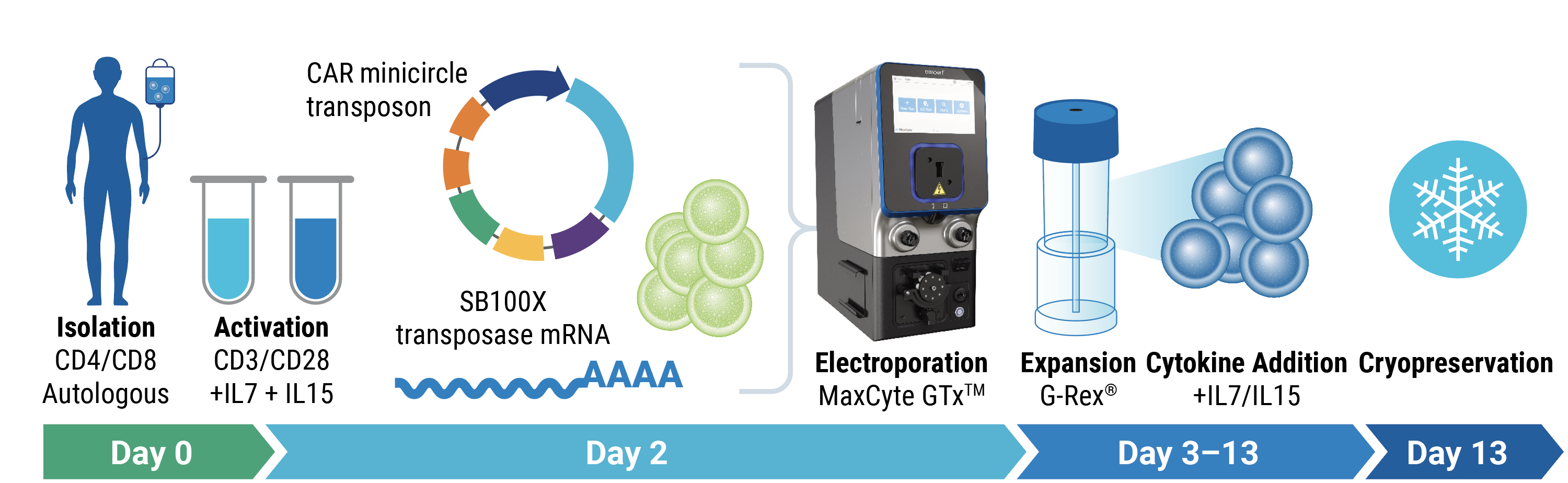
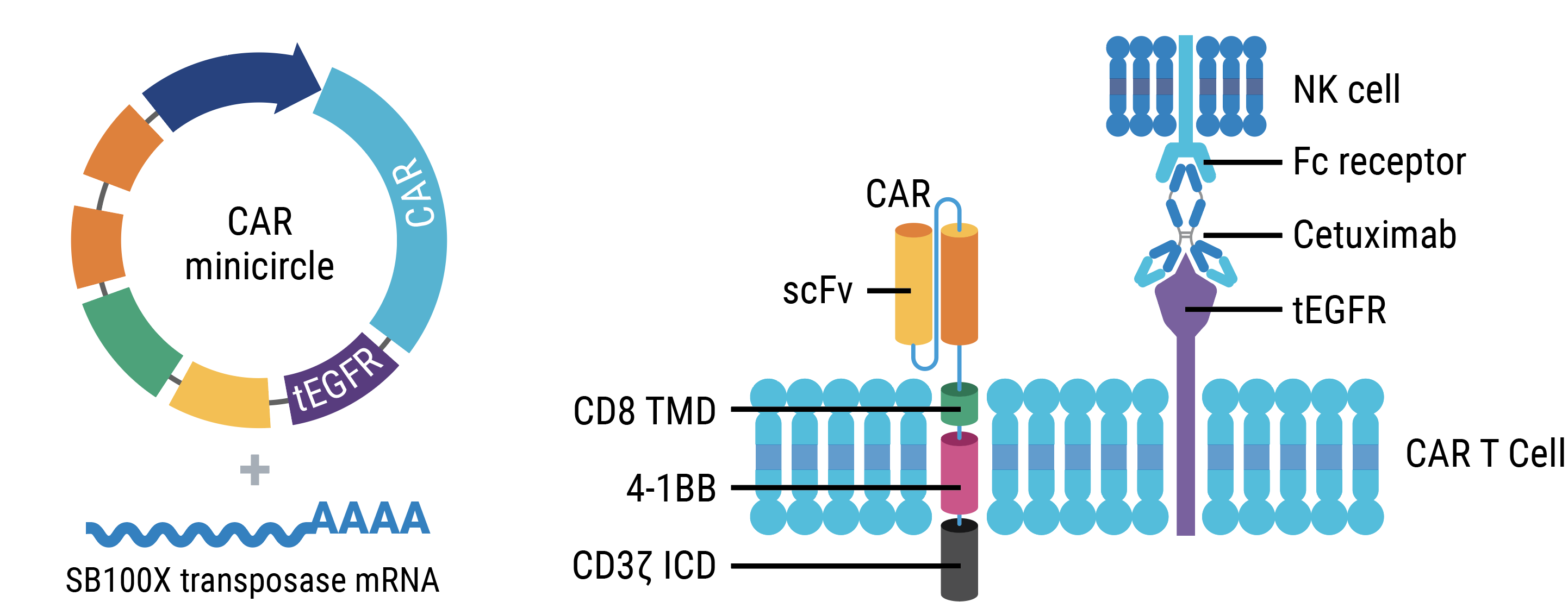
Engineering strategy
T cells were electroporated with SB100X mRNA and transposon minicircle DNA encoding a CAR and truncated human EGFR (tEGFR). Expression of tEGFR was used as a marker for successful transposon insertion and as a safety switch, enabling Transpo-CART cells to be targeted and depleted on treatment with anti-tEGFR antibodies.
Results
MaxCyte electroporation enabled high-efficiency transposition to produce healthy, functional TranspoCART cells.
A
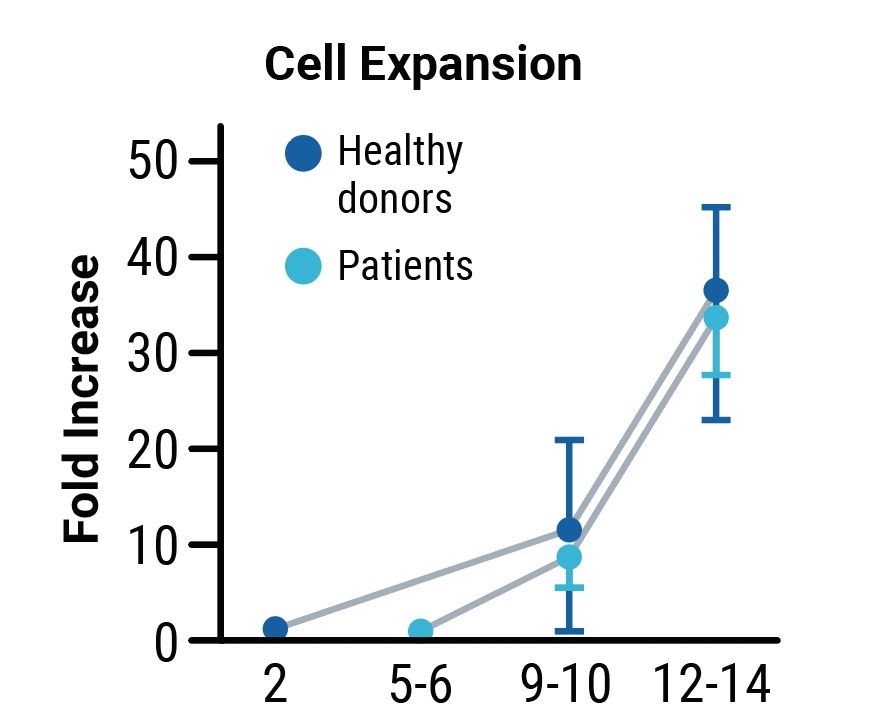
B
C
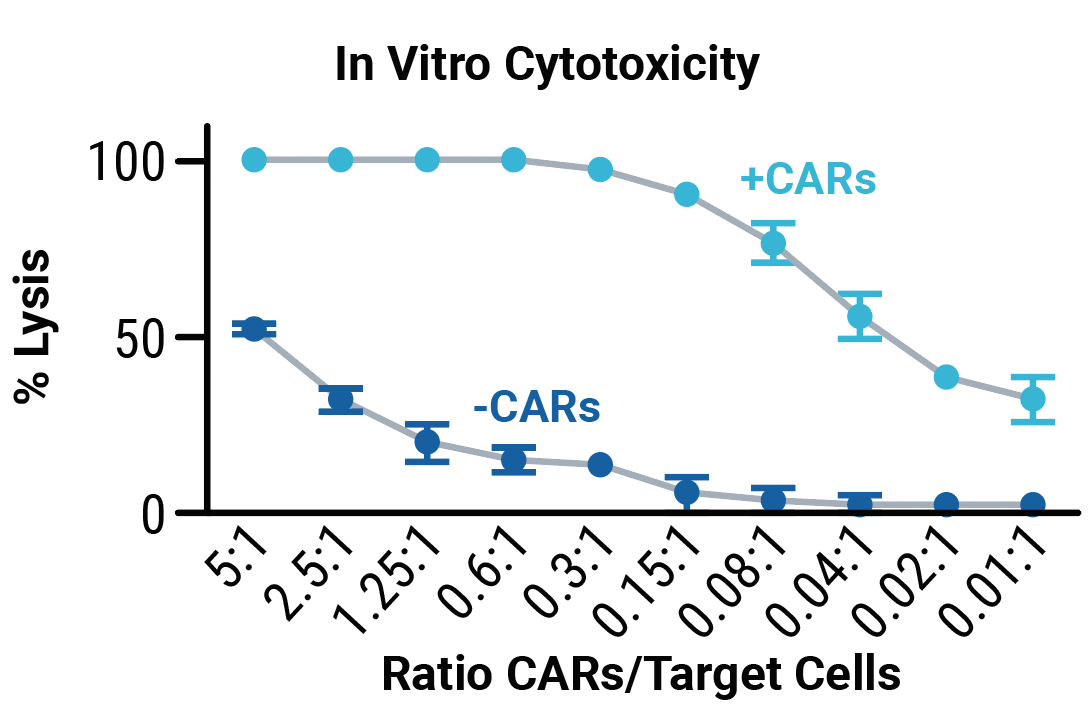
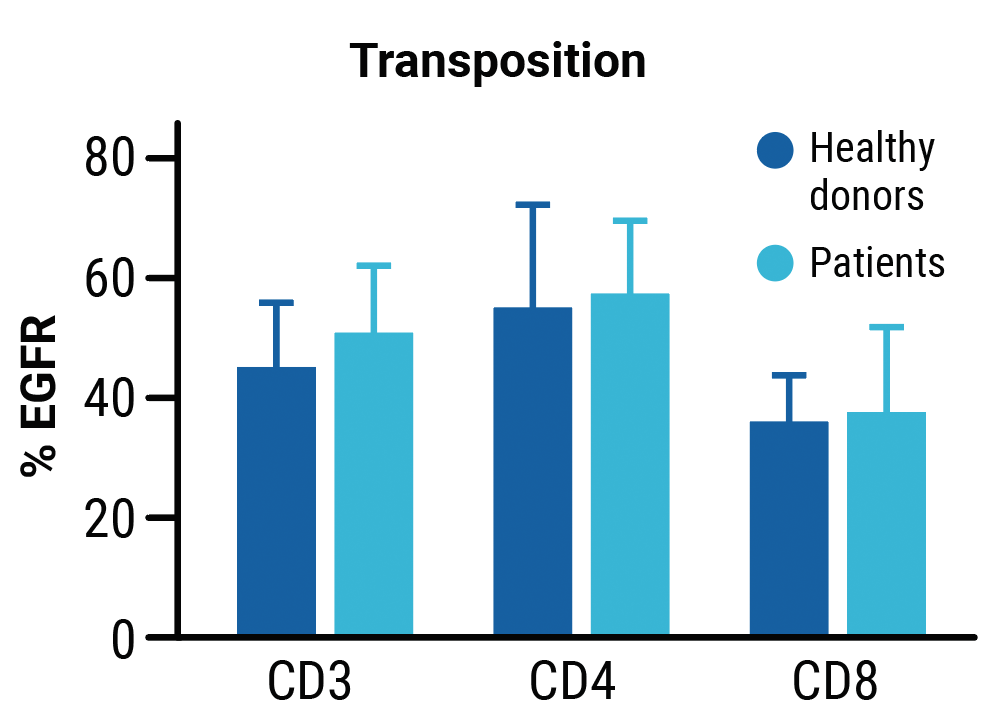
No differences in transpoCART expansion (A) or the rate of transposon insertion (B) were seen between healthy donor and patient samples. TranspoCART cells from patients showed in vitro cytotoxicity against a CAR target-expressing cell line (C).
A transient editing window and tEGFR switch may create a safer therapy.
A
B
C
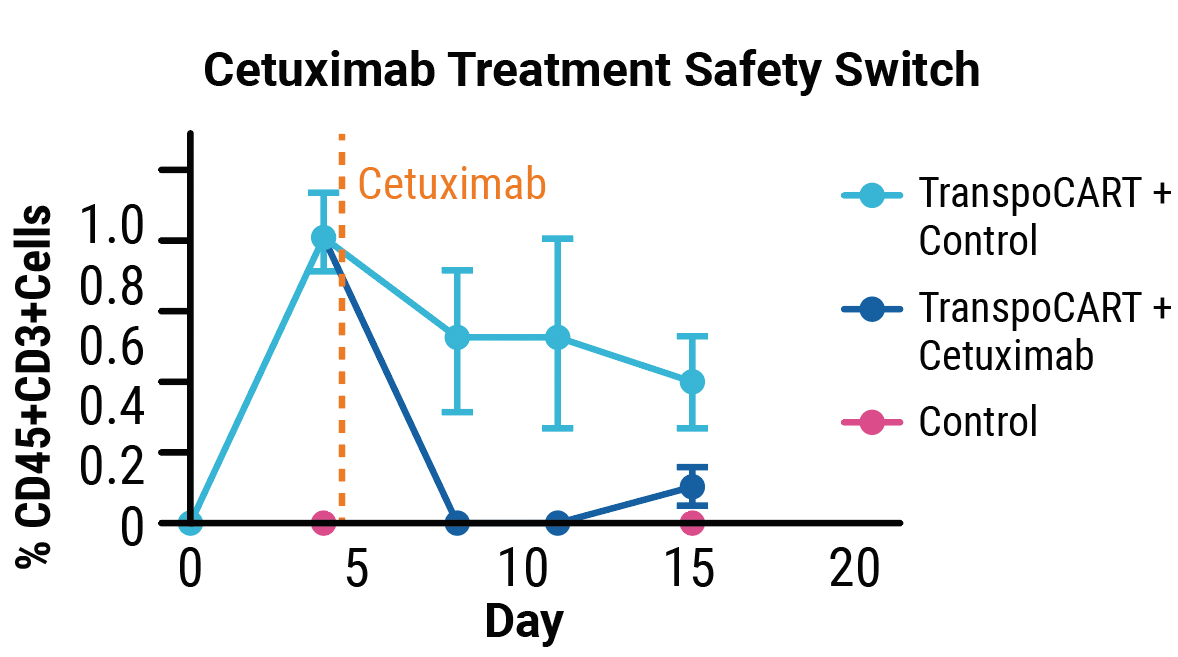
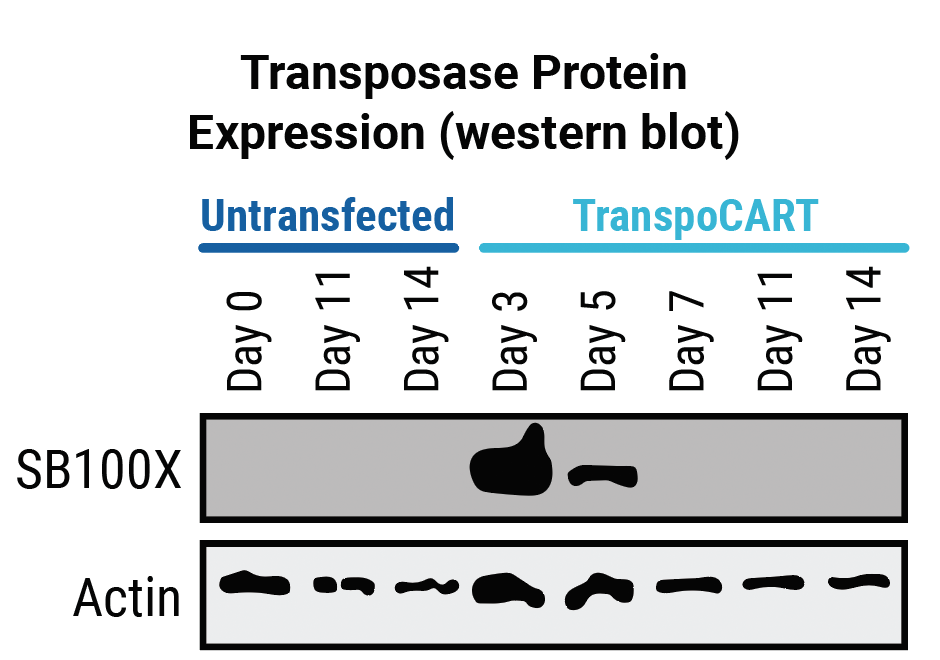
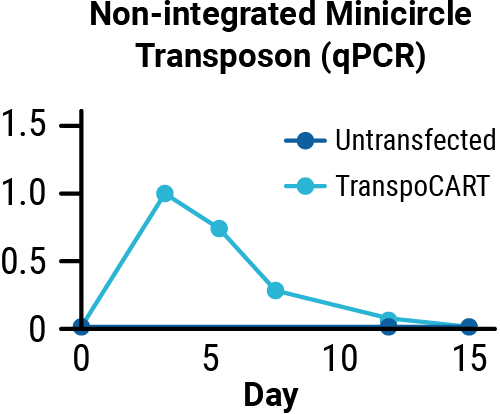
Transposase protein (A) and unintegrated minicircle transposon DNA (B) are undetectable by days 7 and 15 after electroporation. Cetuximab treatment depletes TranspoCART cells in vivo (C).
TranspoCART treatment prolongs survival in a mouse model.
Mice treated with TranspoCART cells generated from healthy donor or patient T cells showed improved survival, with efficacy similar to lentiviral-generated healthy donor CAR T cells.

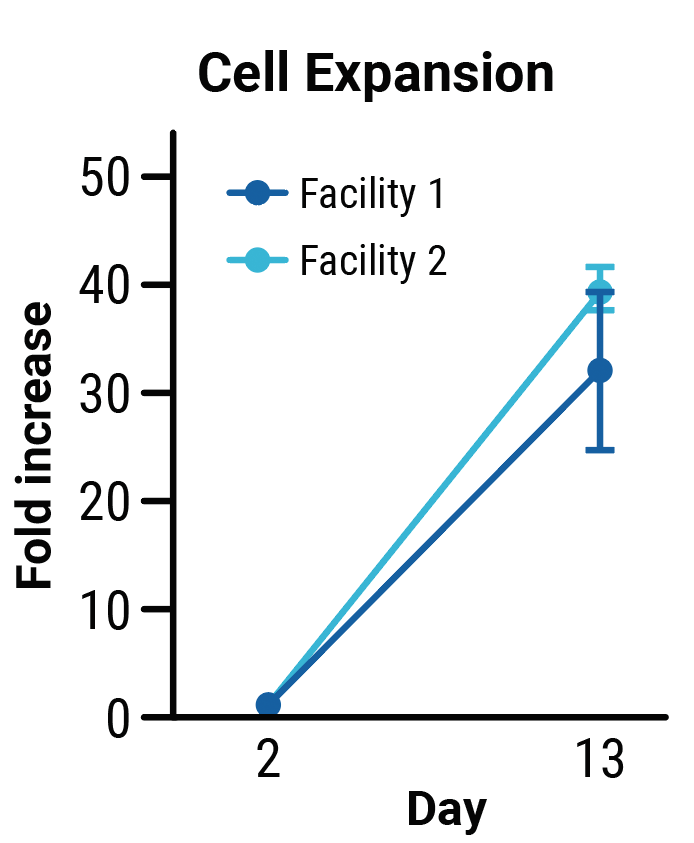
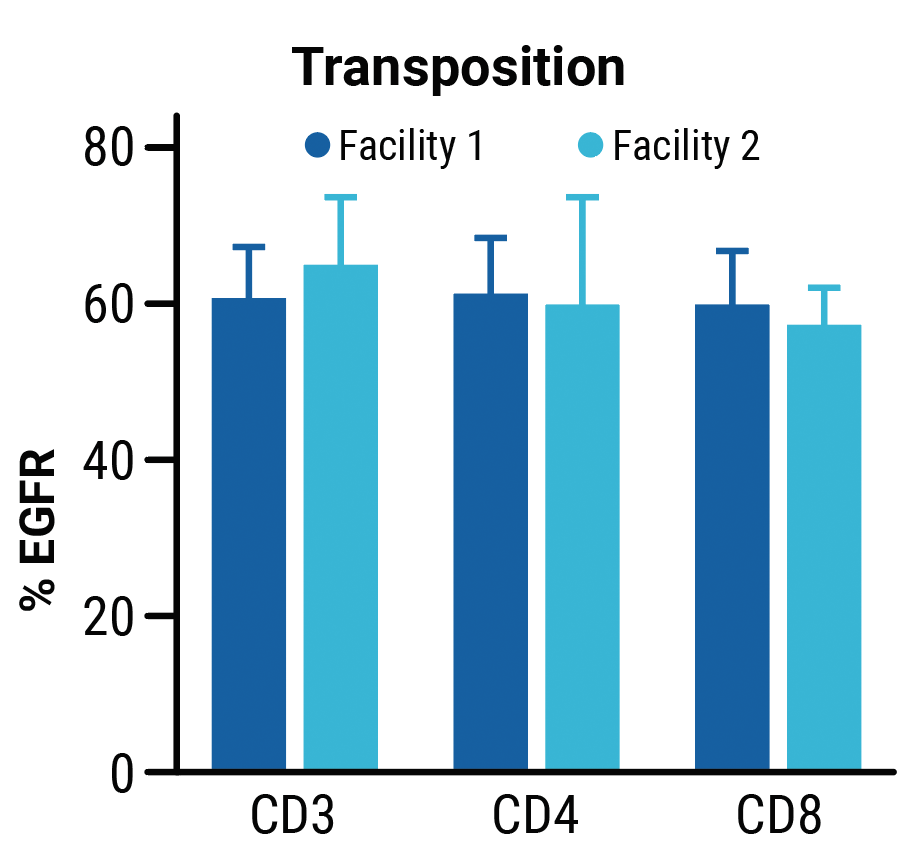
TranspoCART cell production is reproducible across different manufacturing sites.
A
B
C
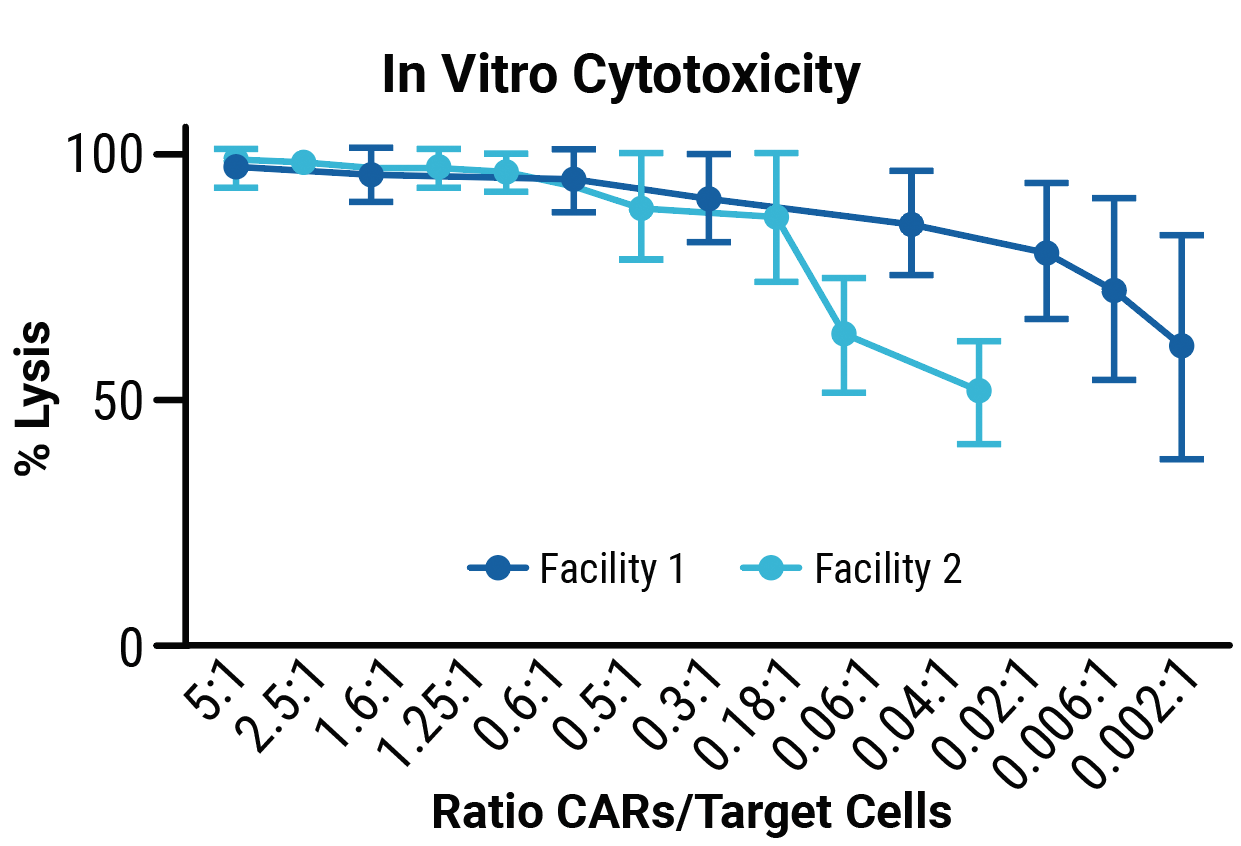
TranspoCART cells produced at two different laboratory sites showed no differences in cell expansion (A) or the efficiency of transposon insertion (B). TranspoCART cells manufactured in both facilities were cytotoxic when cultured with target cells (C).
Summary
- TranspoCART MaxCyte electroporation enabled codelivery of transposase mRNA and CAR minicircle DNA for efficient transposon insertion in healthy donor and autologous patient T cells Reproducible transfection delivering consistent results
- The cGMP-compatible MaxCyte ExPERT GTx® enabled the efficient, reproducible manufacturing of autologous TranspoCART cells
- MaxCyte electroporation enables Sleeping Beauty-mediated gene transfer for a safe, rapid, cost-effective alternative to viral vectors to generate CAR T cell therapies
References
Data generated in collaboration with CIMA/Clinica Universidad de Navarra and CIEMAT. Adapted with permission.