Cell-Based Screening: Accelerating Therapeutic Development
Drug Discovery News
Webinar
June 26, 2025
MaxCyte® shares cell-based screening strategies to overcome barriers in speed, scalability and biological relevance
Therapeutics development benefits substantially from physiologically relevant assays, ideally ones using disease-relevant cells or organoids. Cell-based assays in small-molecule drug discovery have traditionally relied on stable cell lines to deliver consistent assay performance at screening scale. However, stable cell line development can be costly and time consuming. For protein or cell-based immunotherapeutic discovery, phage display is considered the gold standard, but its long selection cycles and complex downstream development can create bottlenecks.
In this DDN webinar, MaxCyte Senior Vice President of Technical Applications and Customer Support James Brady, PhD, discusses how MaxCyte's advanced transfection technology enables the development of faster, more flexible cell-based screening assays, illustrated by case studies and real user datasets.
Key topics covered
-
Benefits and challenges of cell-based assays: Cell-based assays streamline drug discovery by enabling screening and mechanistic studies in a physiologically relevant context. However, conventional transfection technologies may work poorly or inconsistently with relevant cell types.
-
How transient transfection with MaxCyte optimizes assay development: MaxCyte's scalable electroporation platform provides developers the flexibility to work with almost any mammalian cell type and molecular cargo. The MaxCyte instruments are designed to fine-tune assay responses while maintaining desirable cellular phenotypes and membrane biology. Further, MaxCyte's transiently transfected assay-ready cells can replace complicated and costly stable cell lines in assay development and screening.
-
Mammalian display screening for antibody and cell therapy discovery: Also touched on is this emerging mammalian screening application. Mammalian cells, similar to phage or yeast, can be engineered to display a variety of protein formats. Using MaxCyte electroporation for mammalian display gives developers greater ability to identify candidates with either less immunogenicity or more favorable developability as well as greater sequence diversity and library coverage than they could achieve with other transfection platforms.
Watch how to develop faster, more flexible cell-based screening assays

Presenter

Have more questions?
Send your question to one of our cell engineering experts.
Related resources
Transcript
Yuning Wang (moderator): Hello everyone and welcome to today's DDN webinar. I'm Yuning Wang, associate science editor for DDN, and I'll be moderating our discussion.
We have an exciting webinar planned for you today. Our speaker, Dr. James Brady, will discuss how new cell-based screening strategies are helping overcome major barriers in therapeutic discovery. He'll explain how advances in transfection technology and mammalian display systems can streamline assay development and accelerate the identification of promising drug candidates. After the talk, Dr. Brady will join us for a live Q&A session. To sum your questions or comments, we can simply enter them in the q and a chat box on the right side of your screen. We'll do our best to answer as many as we can. So be sure to send in your questions and feel free to ask anything that's on your mind.
I'd like to take this opportunity to thank our webinar sponsor MaxCyte. MaxCyte’s breakthrough transfection technology enables rapid high efficiency delivery of genetic material into virtually any cell type. Their scalable, clinically validated platform helps researchers accelerate timelines and boost productivity in drug discovery, self therapy, and gene editing workflows. Our sponsor has provided us with some helpful handouts related to today's webinar. We can find an access use in our handout section located on the right side of your screen. Also, in the handout section, you'll find your certificate of attendance for participating in today's live event.
And with that, let me introduce our speaker, James Brady. Dr. Brady is the senior vice president of technical applications and customer support at MaxCyte. He has deep expertise in gene and cell therapy, biologics and drug discovery. Before joining MaxCyte, he served as a senior scientist at genetic therapy and as a group leader at MetaMorphix, where he led genetic research programs. Dr. Brady holds a PhD in genetics from Indiana University, completed a postdoctoral fellowship at the National Eye Institute and NIH, and also earned an MBA in finance from John Hopkins University. Dr. Brady, we're so pleased to have you join us today and we look forward to your talk. Let's just take a moment to make sure your slides are up. Great. Dr. Brady, please take it away.
James Brady: Hello, it's my pleasure to speak with you today about MaxCyte’s scalable electroporation technology and its advantages for streamlined cell-based assay development. I want to start with a brief introduction to MaxCyte. Our company was founded a little over 25 years ago with a vision of transforming cells in therapies through the use of our proprietary Flow Electroporation technology, which offers multiple benefits over viral vectors for ex vivo gene delivery.
And our mission from the very beginning has been to enable highly efficient loading of molecules into precious cells from patients while imparting minimal perturbation to either cell viability or cell phenotype. And the therapeutic benefits of MaxCyte’s technology have been validated in many different clinical trials. Most recently, I want to highlight the critical role that our platform has played in manufacturing the first FDA-approved, CRISPR-engineered stem cell therapy for treating sickle cell disease and beta thalassemia. And on the left in this slide, you can see our ExPERT line of instruments. We offer several different instrument models to address the needs of researchers and therapeutic developers, from early stages of discovery through commercial manufacturing. And over the years, we've actually expanded beyond cell therapy into application areas such as bioproduction and drug discovery. But the expertise that we develop by working with these precious primary cells from patients translates in the benefits for users and the drug discovery and buyer production arenas.
Now, today I'm going to focus on how critical features of the MaxCyte platform, including cell and loading agent flexibility, high transfection efficiency, high cell viability, as well as scalability, all provide multiple benefits for assay developers and for drug candidate screening.
I'll begin with an introduction to cell-based assays, focusing on the benefits of cellular screening during therapeutic development, as well as highlighting some common assay related challenges. Next I'll describe how MaxCyte’s electroporation technology addresses many of these assay challenges by providing reproducibly, high transfection efficiency at multiple scales with diverse cell types and loading agents. I'll also highlight the ability to rapidly generate assay-ready cells following large-scale Flow Electroporation, followed by cryopreservation. And to further illustrate these benefits, I'll include a case study on high throughput ion channel screening. I’ll also share a few other data sets from MaxCyte’s customers and end users, concluding with a brief discussion on how MaxCyte electroporation can enable screening via mammalian display for screening protein-based therapeutics.
The overarching advantage of cell-based assays is that they enable screening and mechanistic studies in a physiologically relevant context. So ideally, they allow drug developers to work with cells derived from the tissue type of the therapeutic target. However, there are multiple challenges associated with cell-based assays. Conventional transfection technologies may work poorly or inconsistently with relevant cell types. Complex multi-subunit targets may require optimization of subunit stoichiometry, and also over-expressing targets such as ion channels may cause cell death. And similarly, highly expressed targets are often silenced in stable cell lines, which can lead to expression drift with repeated passaging.
Now, transfection is used to engineer cells for cell-based assays, either by transient expression or by generating stable cell lines. While MaxCyte is used for both transient and stable expression, today I'm going to focus primarily on transient expression of targets and reporters. Transfection plays a key role in all phases of drug development, beginning with target discovery and functional characterization. Transient transfection, or transient expression, offers a streamlined approach to screen multiple target mutations or therapeutic candidates. Moreover, with MaxCyte’s scalable electroporation platform, transient transfection can be used not only to develop assays but also to generate sufficient numbers of cells for high-throughput screening.
I want to touch on a few key considerations related to assay development using transient transfection. Early on, it's best to begin with a series of smaller-scale transfections, usually a few million cells per condition, to identify appropriate cell lines, reagents and detection protocols. And these pilot transfections can also be used to dial in or fine-tune assay responses by varying molecular concentrations, plating densities, as well as detection protocols.
And then larger-scale transfection with tens to hundreds of millions of cells are often used to validate assays, either by confirming assay sensitivity signal, the background ratios, as well as reproducibility. Then, as with assay development using stable cell lines, it's important to obtain robust Z-prime scores, as well as relevant EC50 values and to demonstrate tolerance to vehicles such as DMSO. This is also the time to optimize plating densities or plating conditions and to confirm that assay responses maintained during scale-down to the final high-throughput screening format.
And then finally, in the case of MaxCyte platform, Flow Electroporation with billions of cells can then be used to generate sufficient cell numbers for cryopreservation and high-throughput screening.
I'd like to highlight some of the key features and benefits of the ExPERT electroporation technology that address the challenges and considerations I just discussed. First and foremost, MaxCyte provides high transfection efficiency while maintaining high cell viability with a wide range of immortalized cell lines and primary cells. The technology is compatible with essentially any type of molecular cargo, including large plasmids, and it provides efficient co-transfection with multiple plasmids. This enables efficient expression of either multi-subunit targets or co-expression of targets and reporters.
I should also point out that users don't need to optimize or guess at the best electroporation parameters for their cells. The ExPERT software includes optimized electroporation protocols for a wide range of cell types, so users simply select their cell of interest from a dropdown menu and then they select the scale of transfection from another menu.
Scalability is enabled by our wide range of consumable electroporation chambers that we call processing assemblies. These accommodate anywhere from thousands to billions of cells per sample. Some examples of our processing assemblies are shown in the lower right. At the lower end of the spectrum, we offer multi-well strips, ideal for optimizing plasma ratios or concentrations. And then at the upper end, we have processing assemblies with bags that can hold up to 10 or 20 billion cells per sample. Typically, you can process 20 billion cells in less than 20 minutes.
On the next few slides, I'll provide some examples of assay development and optimization using MaxCyte electroporation. First, to illustrate the point I made earlier about MaxCyte’s versatility, efficiency and enablement of assay development with relevant cell types, these images of cell lines and primary cells that were electroporated with GFP plasmid DNA provide a visual representation of MaxCyte’s ability to provide high transfection efficiency while maintaining cell health in a wide range of mammalian cells as well as insect cells.
Regarding target and loading agent flexibility, here are some examples of the various molecules and products that can be expressed using the MaxCyte platform. The list includes drug targets such as ion channels and GPCRs, receptors, gene editing reagents such as Cas proteins and other nucleases, recombinant proteins including monoclonal antibodies, BiTEs, antigens, enzymes, transcription factors, etc. It's also been used to make viral vectors or virus-like particles as well as vaccines.
Now, earlier I brought up the ability to express complex, multi-subunit drug targets using MaxCyte. In this example, a customer co-transfected HEK cells with four plasmids, which encoded three subunits of a voltage-gated calcium channel, plus an inward-rectifier potassium channel. The transfected cells were then assayed using DI flux on a FLIPR, and you can see a robust assay response that is inhibited by the presence of Conotoxin. So this indicates that these cells are indeed suitable for drug screening.
One major benefit of transient transfection during drug discovery is the ability to screen multiple target variants without investing time and effort to generate separate stable cell lines for each variant. In this example, a pharmaceutical company was screening for compounds to target the TRPV1 ion channel. To ensure relevant data from their animal studies, they wanted to confirm that TRPV1 channels from other species exhibited similar drug responses relative to the human isoform. Rather than generating a series of species-specific TRPV1 cell lines, they used MaxCyte electroporation to transiently express and screen the various species’ isoforms.
Here you can see comparative assay data with the rat and human isoforms. They electroporated three different DNA concentrations for each channel. All of the transfected cells yielded robust assay responses to the agonist and antagonist compounds. And you can see high Z-prime scores for all of the data sets.
Now I want to share a couple of data sets that illustrate assay development with relevant cell types using MaxCyte electroporation. In this first example, a pharma company was developing drugs to target CNS diseases, and they had two drug targets that they wanted to over-express in primary neurons. So hippocampal, ventricular and cortical neurons were isolated from rat embryonic brains, and then those were electroporated either with GFP plasmid to measure transfection efficiency or with two concentrations of expression plasmids that encoded their target proteins. You can see strong GFP expression as well as normal neuronal morphology with the GFP control transfections. And then with both of their target proteins, they were able to obtain robust assay responses that correlated with the DNA concentration. So these data also demonstrate the ability of MaxCyte users to optimize assay responses by varying the amount of DNA in the electroporation reaction.
Here's another example of assay development with physiologically relevant cells. In this case, a drug developer was seeking treatments for cystic fibrosis by screening small molecules to correct or potentiate mutations in the CFTR protein. And their screen was based on a halide flux assay with yellow fluorescent protein, or YFP, as the fluorescence dye sensor. Now, CFTR screening is often conducted with stably transfected fisher rat thyroid cells or A549 cells. Neither cell type is ideal. FRT cells are derived from rats, so they can produce potentially misleading results when you're screening for therapeutics to target proteins in human cells. And then A549 cells, while they're of human origin, they're not of bronchial origin. So therefore, this drug developer wanted to develop a cell-based screen using CFPE cells, which are derived from human bronchial epithelium.
MaxCyte co-transfected these CFPE cells with plasmids encoding the CFTR mutant protein, as well as with YFP. And on the left side you can see YFP expression and the transiently, transfected CFPE cells, which are being compared to stably transfected FRT and A549 reference cell lines. In the upper right, you can see how they were able to optimize their assay response by titrating either the concentration of the YFP plasmid or by varying the relative ratios of the YFT and YFP and CFTR constructs. And then finally, in the lower right, the plot shows that the transfected CFPE cells exhibit comparable assay performance relative to stably transfected FRT and A549 cell lines in the presence of the referenced drug molecule.
Now to reinforce the point I made earlier about dialing in optimal assay windows by titrating the plasma concentration, here's another customer-generated dataset showing how the DNA concentration in the electroporation reaction directly correlated with DI flux assay response in HEK cells, which were transfected with increasing concentrations of a GPCR expression plasmid.
I also want to clarify that when MaxCyte’s technology is used to screen multiple variants or targets, it's usually not necessary to perform a separate DNA titration for every plasmid. In this example, the user was screening potassium channels via automated patch clamping with transiently transfected CHO K1 cells. And you can see on the left, when they transfected or expressed Kv1.3-GFP fusion protein, 80 – 100 micrograms per mil was pretty much the ideal DNA concentration. Also you can see in the photos on the left, because this is a GFP fusion protein, they could use GFP fluorescence to demonstrate high levels of ion channel expression in these cells. And then on the right for Kv1.5, somewhere between 100 – 200 micrograms per mil of DNA gave good current responses. So based on these data, the user could likely lock in 100 micrograms per mil as their standard DNA concentration for screening potassium channels or potassium channel variants with this specific cell type and assay platform.
Now to further fine-tune assay sensitivity, MaxCyte users also have the flexibility to vary the cell plating density post electroporation. And it's important to note that MaxCyte doesn't place any restrictions on either the culture vessels or the assay formats that can be used with transfected cells. In these published data from the Scripps [High-Throughput] Molecular Screening Center, HEK cells transfected with a trip channel or plated in 1536-well plates at four different densities, and as we showed earlier with the DNA concentration, the cell plating number directly correlates with the assay window. In contrast, transfection protocols using lipids or other reagents generally require cells to be plated at a fixed density because a separate transfection reaction usually takes place in each well, and the reagent-based transfection is usually very strongly impacted by plating density.
In addition to providing flexibility for optimizing the assay response, you can see in this quote from the publication’s authors, they've highlighted the advantages of bulk transfection with MaxCyte for overcoming well-to-well inconsistencies and transfection efficiency.
Now, MaxCyte offers a major benefit to assay developers through seamless scalability. Users can transfect thousands to billions of cells at one time without making adjustments to the electroporation settings—and while maintaining transfection efficiency and cell viability. As I touched on earlier, seamless scalability is enabled by a series of single-use consumables that we call processing assemblies or PAs for short. At the lower end, again, we have these multi-well PAs ideal for optimizing loading agent concentrations, plasmid ratios, et cetera. And then after you lock in your cargo concentrations, the reaction can be scaled up to optimize plating, densities and other assay-related variables. And finally, to generate enough cells for screening, you can do the Flow Electroporation and process up to 20 billion cells in a single process. These flow cytometry plots that I'm showing here, these are derived from K562 cells that we transfected with the GFP plasmid using different processing assemblies. These data illustrate the consistency of transfection across our entire portfolio of PAs.
Here's some customer-generated data that illustrate scalability for an actual assay application. On the left U2OS cells were co-transfected with a nuclear hormone receptor expression plasmid and with a luciferase reporter plasmid, and this was done using the OC-400 cuvette-style assembly that could hold 40 million cells. As we showed earlier, the total DNA concentration was varied to optimize the assay window. And then after determining the optimal DNA concentration, the transfection process was scaled up using Flow Electroporation with the CL-2 PA in 1 billion cells. Here you see a comparison of static electroporation with 40 million cells versus Flow Electroporation with 1 billion cells, and you can see a very comparable assay response in the two sets of cells.
By performing large-scale electroporation and then aliquoting and cryopreserving the cells, MaxCyte users have the ability to generate assay-ready cells that can be used for drug discovery and other applications. And as I just described, after optimizing electroporation conditions and assay parameters at small-scale, Flow Electroporation can scale up to 20 billion cells to generate assay-ready cells that are suitable for a wide range of screening applications, including automated patch clamping, DI flux and reporter assays. And aside from dramatically reducing time and labor commitment compared to making stable cell lines by performing repeated, independent transient transfection, assay-ready cells also eliminate problems with expression drift that can occur after repeated passaging of stably transfected cells. Furthermore, assay-ready cells produced via transient transfection provide significant advantages for developing complex assays that require co-transfection or co-expression of target and reporter proteins, or simultaneous expression of multiple target subunits. And then finally, it's much simpler to express toxic targets via transient transfection compared to alternative approaches using stable cell lines, which may require, for example, inducible expression systems.
Now, transfection cells can be plated and used for screening immediately following large-scale Flow Electroporation; alternatively, the bulk transfected cells can be aliquoted into multiple vials and then cryopreserved to generate cells for future screening needs. And there are a few different approaches that are commonly used for post electroporation cryopreservation. Some US users prefer to free cells immediately after electroporation. In this case, a brief resting step before freezing may help to maximize viability. But alternatively, other users choose to cryopreserve cells one- or two-days post electroporation. The advantage of delayed freezing is that cells are cryopreserved when target expression is high. So this allows users to perform assays with the frozen cells shortly after thawing.
Virtually any cryopreservation medium or freezing protocol can be used with MaxCyte-transfected cells. Regardless of the cryopreservation protocol, the primary benefit to degenerating cryopreserved cells via large-scale electroporation is that consistent assay performance is assured among every vial of frozen cells because each vial contain cells from the same bulk transfection. So this eliminates inconsistency in the transfection process as a potential source of assay variability. And these luciferase reporter data generated with transiently transfected Jurkat cells over assay pre- and post-freezing demonstrate that assay performance is indeed maintained following cryopreservation and thawing.
MaxCyte’s website has multiple case studies with assay-ready cells that are based on published data from our customers and partners. These case studies can be downloaded by visiting the URL at the bottom of this slide. In the next few slides, I'd like to share some data from one of these case studies.
This particular case study describes the development, validation and application of a method for high-throughput screening of ion channels using automated patch clamp recordings with assay-ready cells. The data I'm sharing today are from a publication that came out of the laboratory of Dr. Al George at Northwestern University. The George Lab focuses on genetic disorders that are caused by voltage-gated ion channel mutations. In this particular publication, they were characterizing variants of the KCNQ2 potassium channels that are associated with epilepsy. KCNQ2 can assemble into homotetramers, but it also co-assembles with KCNQ3.
And most pathogenic KCNQ2 variants are heterozygous, so therefore, the George Lab used MaxCyte to transiently transfect KCNQ2 variants into a CHO cell line that stably expresses wild-type human KCNQ3. And some of their key study goals were to generate a system for studying mechanisms of drug action and to identify target populations for emerging precision epilepsy therapies.
The authors tested 81 KCNQ2 variants. Most of them were homozygous and heterozygous channels, so they made over 150 assay-ready cell banks showing this workflow. And obviously it's much easier to make 150 sets of transiently transfected cells compared to generating 150 separate stable cell lines. And then the assay-ready cells were prepared and cryopreserved in three days following this simple workflow. To mimic homozygosity, CHO-Q3 cells were transfected with either wild type or variant plasmids. And then to mimic heterozygosity, cells were co-transfected with expression plasmids encoding wild-type and variants on a one-to-one ratio. And then post cryopreservation, cells were thawed, and the functional assays were performed by high-throughput automated patch clamping as shown here.
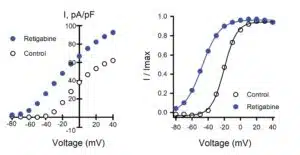
In this figure, the author's tested 39 epilepsy-associated variants that had not previously been investigated. They tested variants that were associated with either benign familial neonatal epilepsy, shown in blue, or with developmental and epileptic and encephalopathy, shown in red. They also looked at one variant with an unclear phenotype, shown in black.
And the researchers tested all variants as homozygous and heterozygous channels, so I'm showing only the heterozygous data here. The authors recorded wholesale currents and calculated differences in the peak density as well as the half-activation voltage compared to data that were generated for wild-type channels that were assayed parallel.
And they identified several dominant negative variants and found many variants that exhibited significant shifts in the half-activation voltage. The authors’ assay also analyzed the response to retigabine, an approved anticonvulsive drug in both homozygous and heterozygous variants.
Data from homozygous screening is not shown here, but those data enabled identification and mapping of several variant residues that has strong effects on ion channel function that cannot be overcome with tine treatment in the homozygous state.
So to summarize this case study, the George Lab is creating a large number of potassium channel variants using MaxCyte’s electroporation platform to generate assay-ready cells. Now, this process allowed them to avoid the extensive time and effort that would be needed to generate many different stable cell lines for each variant. Plus, the high transfection efficiency and high viability of the transfected cells resulted in pharmacological relevant data generation. Now before I conclude, I'd also like to touch on an emerging and impactful screening application for MaxCyte’s electroporation technology, which is mammalian display screening for antibody and cell therapy discovery.
Now, mammalian display is a protein engineering and screening approach based on the presentation of proteins, including antibodies, antibody-derived proteins and receptors. On the surface of mammalian cells, it's conceptually similar to phage or yeast display, but by expressing candidates on the surface of mammalian cells, biologics can be discovered and optimized in a system that closely mimics native human cellular conditions, including preferred protein processing structure and post translational modification.
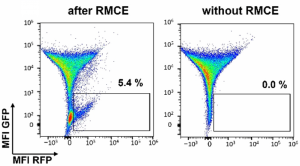
The workflow for a mammalian display screening with MaxCyte is straightforward. As with assay development for drug target screening, the cells of interest are suspended in MaxCyte’s electroporation buffer, along with a library of expression plasmas that encode target proteins. The expression contracts are generally designed by joining the functional protein domain to a membrane target domain. This allows the proteins to be anchored in the cytoplasmic membrane with the antigen or binding domains expressed on the surface of the cell. And then, typically, a recombinase or nuclease plasmid or mRNA is co-transfected with the library to yield targeted insertion of the candidate genes into a specific locus. This is to ensure that each cell expresses only a single-candidate molecule. Post electroporation, transfected cells are often screened by fluorescent activated cell sorting, following incubation with either a labeled antigen or some other ligand. And then finally, DNA is isolated from the sorted cells to determine the sequence of the binder molecule.
While mammalian display was originally developed for antibody discovery, modern drug discovery is moving beyond traditional IgGs to novel antibody formats as well as to cell therapy. So mammalian cells can actually be engineered to display a variety of different protein formats, such as those shown here.
I don't have time to present data on the use of MaxCyte’s platform for mammalian display, but our website contains references for peer-reviewed publications as well as links to case studies that describe multiple examples of mammalian display for antibody as well as for TCR screening in CHO and HEK cells using MaxCyte electroporation. And these case studies and publications highlight a number of key advantages to using MaxCyte electroporation for mammalian display screens.
First, developers have greater ability to identify candidates with either less immunogenicity or more favorable developability. MaxCyte also provides users with a simplified workflow in which a single large-scale transfection process can yield libraries potentially with up to a billion different sequences. So MaxCyte users or developers have much greater sequence diversity and library coverage than they could achieve with other transfection platforms while also achieving greater reproducibility and consistency. Overall, MaxCyte makes it possible to discover relevant candidates with fewer resources and less time.
There's a lot more data I could share about the advantages of MaxCyte’s scalable electroporation technology for cell-based assay development and candidate screening. Obviously, I don't have time to cover all these capabilities, but if there are specific applications that I didn't cover today, please reach out to me via email or visit our website, maxcyte.com.
Before I conclude and take questions, I want to reiterate some of the key features and benefits of the MaxCyte platform for assay developers. Namely, highly efficient transfection that provides the flexibility to work with almost any mammalian cell type and molecular cargo suitable for screening complex multi-subunit targets and toxic proteins; the ability to fine-tune assay responses while maintaining desirable cellular phenotypes and membrane biology; avoiding the time and expense of stable cell line generation; and, finally, seamless scalability, which provides the ability to generate assay-ready cells, as well as to screen large libraries with greater sequence-diversity building via mammalian display.
I'd like to thank everyone for joining today's presentation, and I am happy to address or answer questions.
Yuning Wang: Thank you so much for that great presentation, Dr. Brady. Let's move on to our Q&A session. As a reminder to our audience, you can still submit your questions in the Q&A chat box on the right side of your screen, even when we're talking.
The first question is, what factors have the greatest influence on assay performance with MaxCyte-electroporated cells?
James Brady:That's a great question. Obviously, with electroporation, you're delivering the same electrical parameters every time you hit the button on the instrument. The transfection process itself isn't really a variable, but the quality of the cells that you're working with as well as the quality of the plasmids or whatever you put into the cell, those are the two biggest variables in the process.
For example, in the case of the cells, for cell lines, we recommend using relatively low-passage cells. The cell should not be overgrown. You want viability to be high, of course. And in the case of loading agents, which are typically plasmids for cell-based assays, you want low endotoxin levels. We recommend suspending the plasmids in water instead of TE buffer. But also in the case of ion channels, which can have repetitive subunits, you want to run those on a gel to make sure a recombination hasn't taken place. But if you have healthy cells and good quality DNA, your results are likely to be very good as well.
Yuning Wang: That's really helpful. Thank you for breaking that down. Let's move to our next question.
Can you explain in more detail why electroporation is preferable to other methods of transient transfection for cell-based assay development?
James Brady: I touched on some of these points during my presentation, but again, as I just mentioned my previous answer, the consistency of transfection efficiency is much higher with electroporation because you don't, for example, with a chemical-based transfection method such as lipids, you can have batch-to-batch variability in the quality of the reagents. There's a step where you have to complex the lipid with the DNA, there’s variability in that process. Then the DNA has to get into the cell, which is a variable process in itself. And so with electroporation, you have consistent transfection efficiency. But the key point to keep in mind is that you're delivering the DNA all at once. So that's actually a big benefit if you're trying to time your assay or you're trying to eliminate that well variability. And with MaxCyte, the ability to scale up the process without sacrificing efficiency or losing anything in terms of performance is another key advantage.
Yuning Wang: That makes a lot of sense. Thanks for clarifying those advantages.
The next question is, what is the optimal time point post electroporation for performing cell-based assays?
James Brady: That somewhat depends on the cell type and the assay. It’s usually in the 24-to-48-hour windows when you get peak assay activity. Typically for a reporter assay 24 hours is usually the peak time, somewhere between 20 and 40 hours, for a luciferase reporter assay, for example. Interestingly, for ion channel assays, what a lot of our customers like to do is they'll incubate the cells at 37 degrees for anywhere from six to 24 hours, and then they'll lower the temperature to 28 degrees for another 18 to 20 hours. By dropping the temperature, what they found is that that actually gives time for more ion channels to accumulate on the cell surface. In that case, maybe 48 hours could be the preferred time point for assaying the cells, but usually that 24-to-48-hour window works well for pretty much any assay.
As I touched on earlier too, if you're cryopreserving the cells, you can take that into account. Some people like to freeze right after electroporation, which is simpler. But then when you thaw out the cells, you want to give them a day or so for that target or the different molecules to express. The alternative approach is to freeze the cells when the target is expressed at its peak, and then as soon as you thaw those cells out, as soon as they have an hour or so to recover, you can start running your assays.
Yuning Wang: Great. Thank you for sharing those guidelines. We have a couple of more questions from the audience on the graph of light versus time. I think that's one of your slides. Is it light energy, visible light?
James Brady: A lot of the assays that our customer use are luciferase assays. So those are typically performed on a plate reader that measures luminescence or, in some cases, fluorescence.
Yuning Wang: Another question is [about] heat in proteins—is that a problem with defrosting the cryogenic compounds?
James Brady:These are cell-based assays rather than protein-based assays. Our users typically follow standard cryopreservation protocols as well as standard thawing protocols. I guess the general rule of thumb is you want to cryopreserve slowly and then thaw quickly, but otherwise, we don't place any restrictions on, say, cryopreservation medium. Some people use control rate freezers, but you can also use the Mr. Frosty or the ethanol-jacketed freezing methods. So no real tricks or concerns about the freezing process or the thawing.
Yuning Wang: Sounds good. Thank you again for answering the questions. And that brings us to the end our session for today. If you have any additional questions for our speaker, please feel free to reach out to Dr. Brady directly. His contact details are shown on the screen now. As a reminder, the webinar will be archived on the DDN website, and you’ll receive an email notifying you when the webinar is available on demand. On behalf of DDN, I would like to thank our speaker, Dr. Brady, and our sponsor MaxCyte and, of course, thanks to you for listening.