Cell-Based Assay Development
Developing novel cell-based assays for research or high-throughput screening typically requires cell lines that stably express assay components, including protein subunits and functional enzymes, or purchasing commercially available stable cell lines or assay-ready cells.
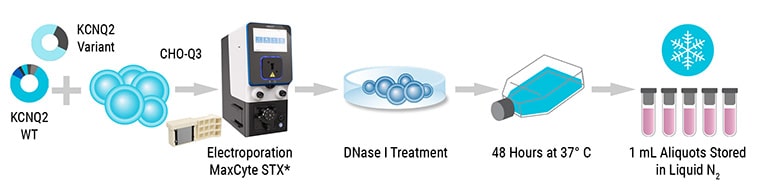
MaxCyte® electroporation enables you to adopt the right approach for each new project; make a single batch of transiently transfected cells for a large, one-off screening campaign, produce assay-ready cells to aliquot and cryopreserve to use when needed, or even develop a stable cell line in the best cell type for your target.
Electroporation for cell-based assays
With MaxCyte technology and support, you can use transiently transfected cells for assay development and screening, saving time and money and providing the flexibility to get precisely the assay you want. Transient transfection with MaxCyte electroporation empowers you with:
- Cell type, target and assay flexibility
- Significantly faster assay development compared to stable cell line generation
- Simplified assay development for toxic targets
- Optimal assays kinetics with efficient concurrent co-transfection of multiple plasmids
- Ability to fine-tune assay sensitivity by varying DNA concentration and plating density
- Flexibility to cryopreserve and plate cells in any plate format
- Improved well-to-well consistency
- Minimal impact on cell health and membrane biology
Together, we can make your assay simple
With defined protocols and expert support, MaxCyte takes the guesswork out of cell-based assay development. Simply test different plasmids and plasmid ratios, DNA concentrations and cell plating densities to fine-tune assay sensitivity using small-scale electroporation. Once conditions are optimized, you can choose to develop a stable cell line or produce a large batch of transiently transfected assay-ready cells.
Transiently transfected cells can express complex, multi-subunit and even toxic proteins while maintaining cell health and membrane integrity. The ability to transfect up to 20 billion cells in one reaction makes producing your own assay or assay-ready cells simple.
Applications include:
Accelerate and de-risk small molecule discovery
Using physiologically relevant cells for assay development delivers the best results, but those cells are not always simple to engineer. MaxCyte's versatile electroporation technology enables you to transfect virtually any cell type rapidly and efficiently, so you don’t have to choose between the best cell and the easiest cell.
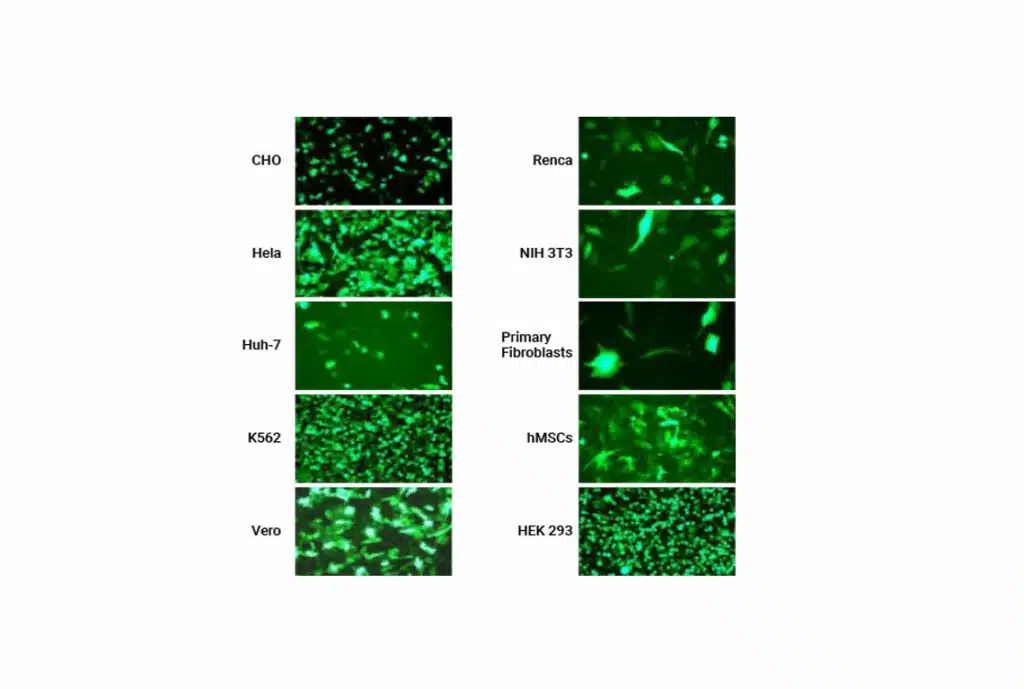
Transfect physiologically relevant cell types with high efficiency and cell viability.
Transition seamlessly from assay development to high-throughput screening
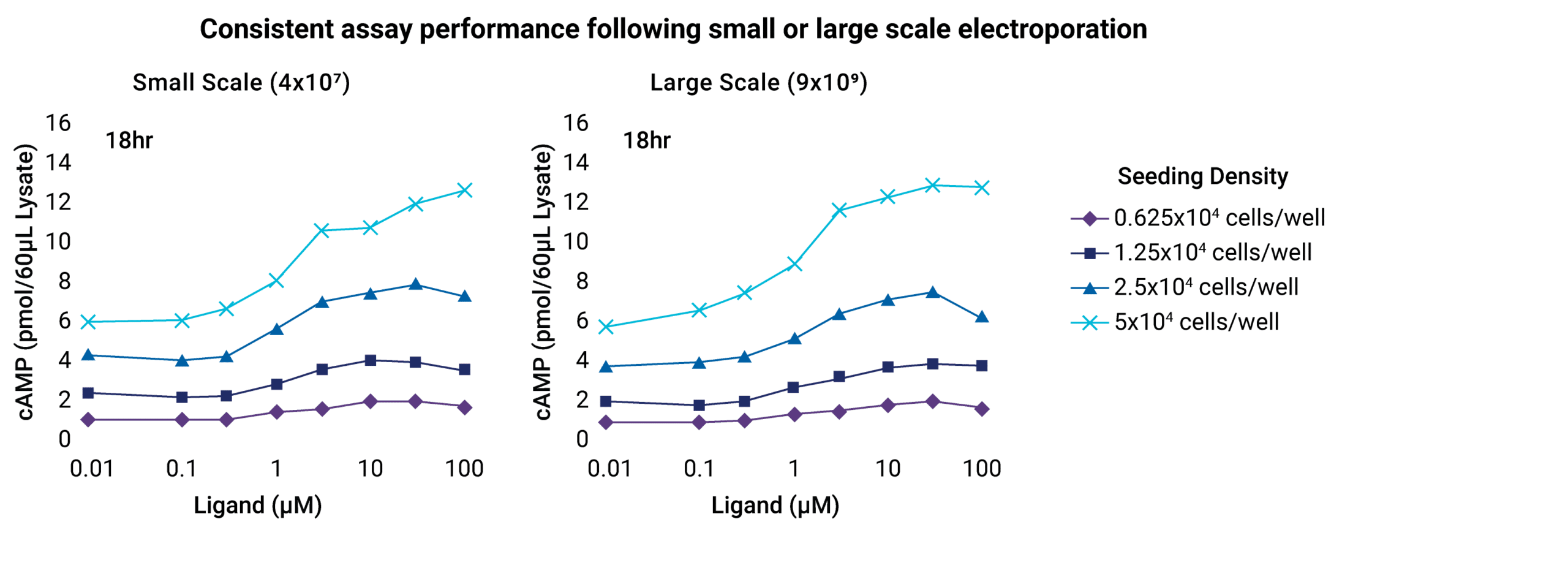
Consistent assay performance following small- or large-scale electroporation.
Because you only want to optimize assay conditions once, MaxCyte® electroporation delivers the same high-efficiency transfection in 75,000 cells or 20 billion cells—and everything in between—without reoptimization. Transiently transfected cells can be used immediately in cell-based assays or cryopreserved for future high-throughput screening.
Resources
Electroporation systems
The MaxCyte ExPERT STx® is the next generation of the industry’s leading electroporation technology for seamless transition from R&D to manufacturing scale. The STx enables large-scale transfection of virtually any cell type for the rapid development of cell-based assays without the need for stable cell lines.


Reagents and processing assemblies
MaxCyte’s consumable products provide a variety of options for project scale and throughput from discovery through manufacturing using a single platform. Our range of processing assemblies allows users to transfect a variety of sample volumes to meet specific application needs. MaxCyte’s universal electroporation buffer is animal-derived, component free and safe for all cell types ensuring consistent, high-performance transfection.
Ready to learn more about our technology?
Ask us how electroporation can streamline development of cell-based assays.