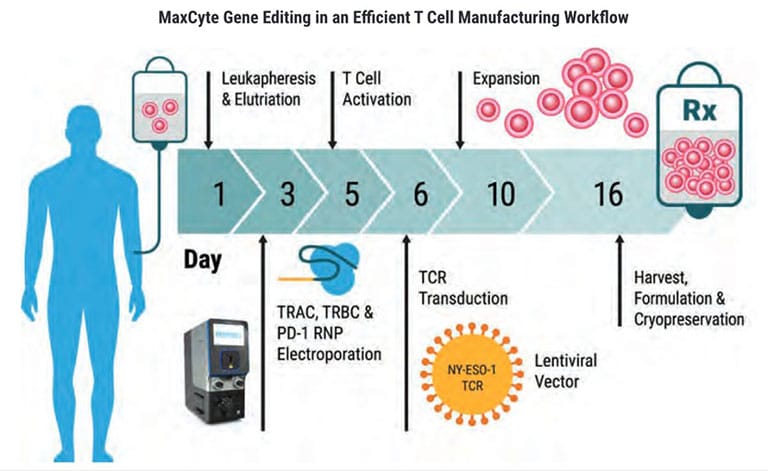
ExPERT GTx®
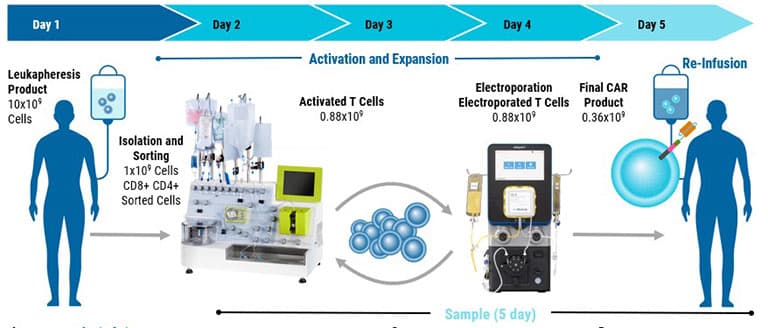
From product development to translation into clinical trials, GTx’s clinically validated scalable electroporation technology is equipped to handle your most complex cellular engineering demands.
Rapidly transfect up to 20 billion cells.
Move forward with 21 CFR Part 11 enabled software.
Develop quickly with an established regulatory path supported by a FDA Master File.
Manufacture confidently with closed, cGMP-compliant, ISO-certified and CE-marked products.
Enjoy MaxCyte’s proprietary Flow ElectroporationⓇ technology.
Product features & benefits
Consistent, reproducible results: Achieve robust transfection across various donors and manufacturing sites.
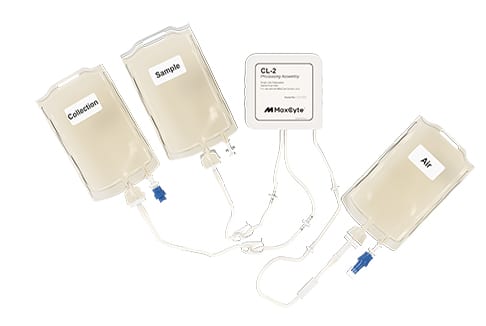
Seamless tech transfer: Simplified instrument and processing assembly setup reduce operator-related errors.
Open connectivity to DCS: Seamless real-time integration with Distributed Control Systems (e.g., DeltaV™) for consistent process control, data collection, and improved batch records.
Time synchronization: Unified timestamps across devices for enhanced compliance, data integrity and simpler audits.
Small footprint: Minimize space requirements in the manufacturing suite.
Pre-optimized protocols: Over 80 cell types supported, saving time on electroporation parameter optimization.
Fast processing: 8 mL/min throughput helps maintain cell health.
High cell concentrations: Process 0.5–2×10^8 cells/mL for both autologous and allogeneic workflows.
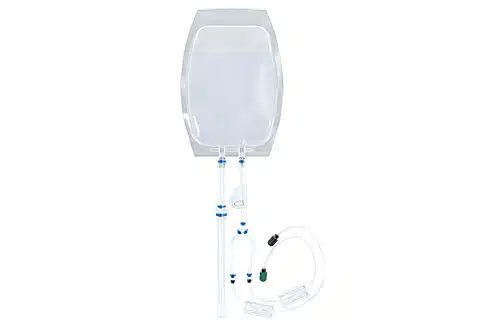
Scalability: Flexible processing assemblies handle volumes from 20 µL to 100 mL.
Maximize data management efficiency with open connectivity
The ExPERT software seamlessly integrates with distributed control systems (DCS) for real-time monitoring and data collection.
- Enables consistent process control and improved batch records
- Validated connectivity with DeltaV™ DCS


Choosing MaxCyte® gives you more than just an instrument — partner with an expert in cell engineering with the knowledge to support you during every stage of your journey of discovery
Supporting products
Compatible consumables & accessories
Research applications
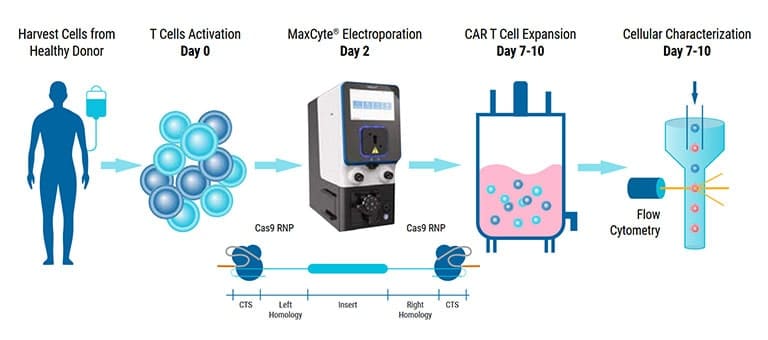
Learn how scientists are using GTx™
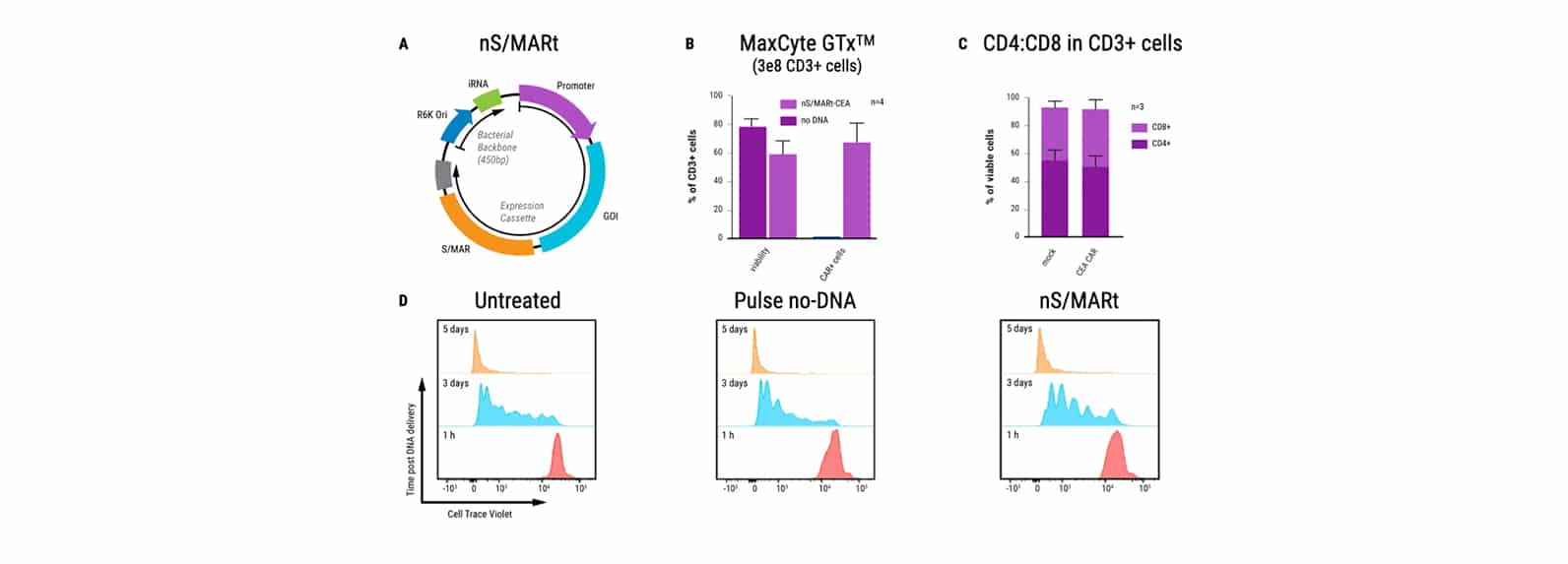
An alternative vector platform for the manufacture of recombinant T cells for autologous T cell immunotherapy
Dr. Harbottle discusses how nS/MARt DNA vectors can provide robust transgene expression in every cell and model system available.