The 13th Antibody Industrial Symposium (AIS) 2025 brings together leaders from academia and industry to address the latest advancements, tools and challenges in therapeutic antibody and biologic drug development. Jointly organized by LabEx MAbImprove and MabDesign, the event features 11 expert-driven sessions, dynamic pitch talks and extensive networking opportunities—all focused on accelerating next-generation biotherapeutics.
MaxCyte® is pleased to participate in this year’s symposium, where we will highlight our GMP-compliant Flow Electroporation® technology, a scalable non-viral delivery platform that supports rapid, high-efficiency transfection across multiple cell types. Our technology is designed to streamline the entire therapeutic development continuum—from early discovery through to clinical and commercial manufacturing.
Poster presentation
Innovative approaches to reducing time, costs and risk of failure in biologic drug discovery and development
Wednesday, June 25, 2025 from 7:00 to 8:30 p.m. CEST
Since the approval of the first therapeutic monoclonal antibody, the field of biologics has rapidly expanded, with numerous novel formats and applications. However, the pace of new biologic approvals has not matched this growth. Significant challenges remain, including lengthy development timelines, high costs and high attrition rates of lead candidates.
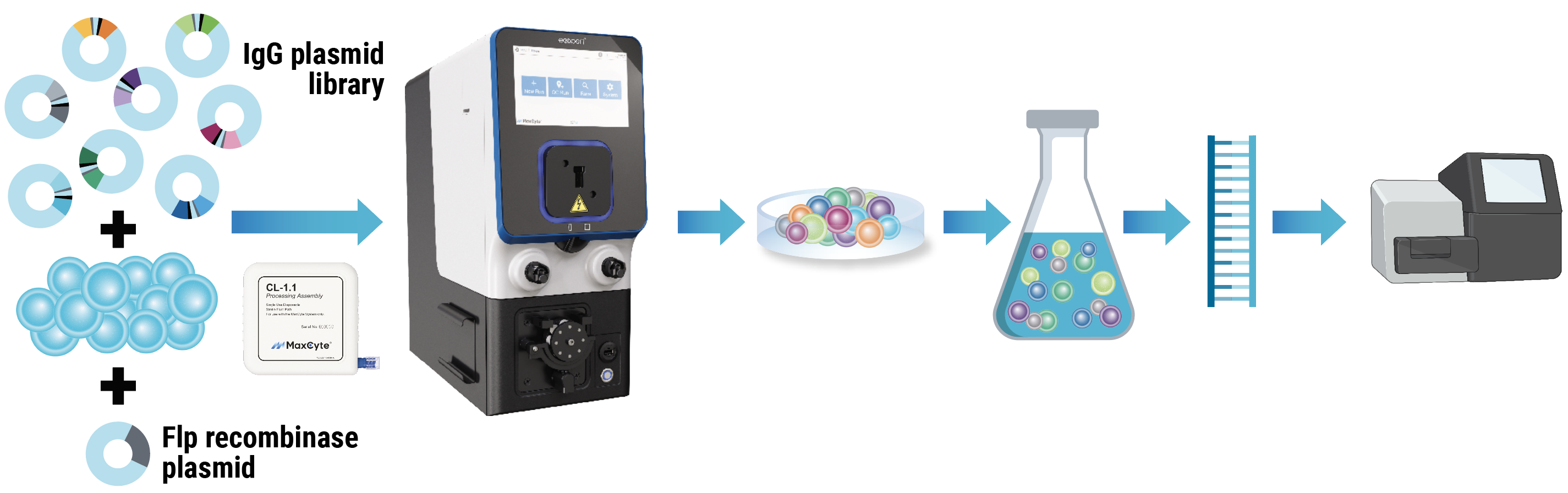
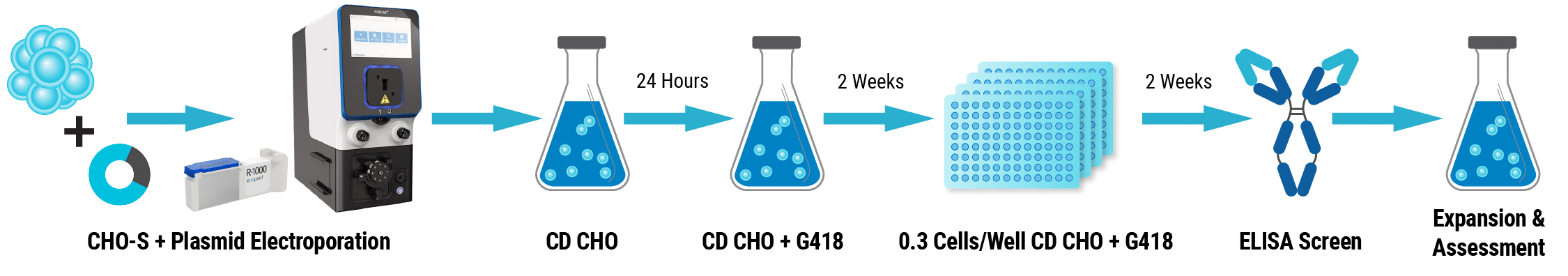
Here, we present strategies to streamline every stage of biologics discovery and development. We discuss methods such as mammalian display to identify candidates that exhibit high affinity and specificity, along with optimal developability and a reduced likelihood of failure. Furthermore, we explore high-throughput transient protein expression for rapid screening with reduced downstream re-optimization, large-scale transient protein expression in manufacturing cell lines, and expedited development of stable pools and cell lines to speed up preclinical development.
MaxCyte’s electroporation technology is uniquely equipped to support all these innovative strategies, enabling optimized therapeutic antibody development workflows from concept to commercialization.
Presenter