CAR T Cell Manufacturing
Partner with MaxCyte® to navigate the challenges of CAR T cell manufacturing. With us, you can achieve highly efficient delivery of gene editing constructs to difficult-to-transfect primary T cells while maintaining cell viability. With our scalable electroporation technology, you can streamline the development of CAR T cell therapy from concept to commercialization.
Introduction
The development of CAR T cell therapies has transformed the way we approach treating cancer. This innovative strategy uses engineered immune cells to target and eradicate cancer. This process involves several crucial steps to ensure CAR T cell efficacy and safety.
How are CAR T cells manufactured?
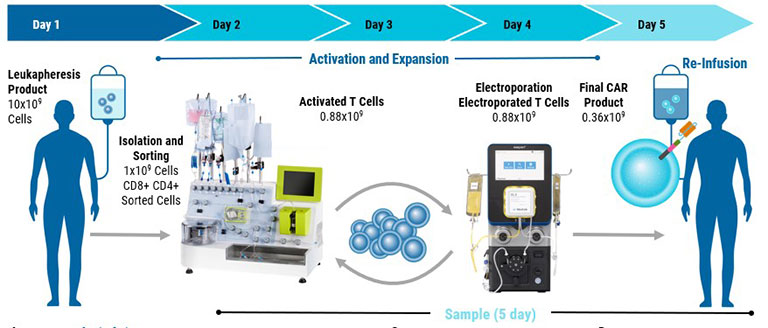
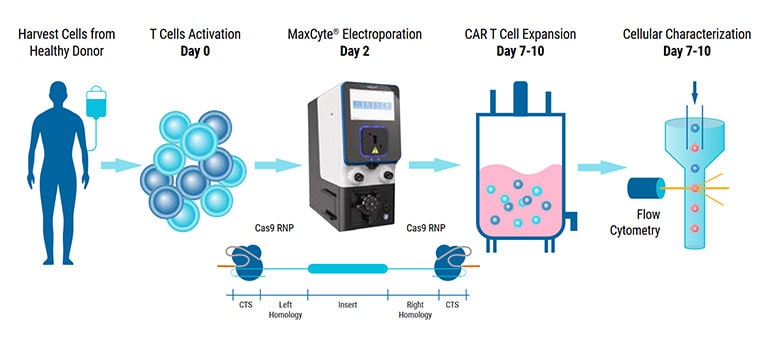
To begin the T cell manufacturing process, a patient's T cells are collected through leukapheresis. These cells then undergo genetic modification where genes encoding CAR receptors are introduced into the T cells using a variety of delivery mechanisms such as electroporation.
Once the genetic editing is complete, the CAR T cells are expanded by providing the cells with essential growth factors and nutrients to support their proliferation. Rigorous quality control tests are conducted to evaluate the potency, purity, and safety of the engineered CAR T cells before they are given to the patient.
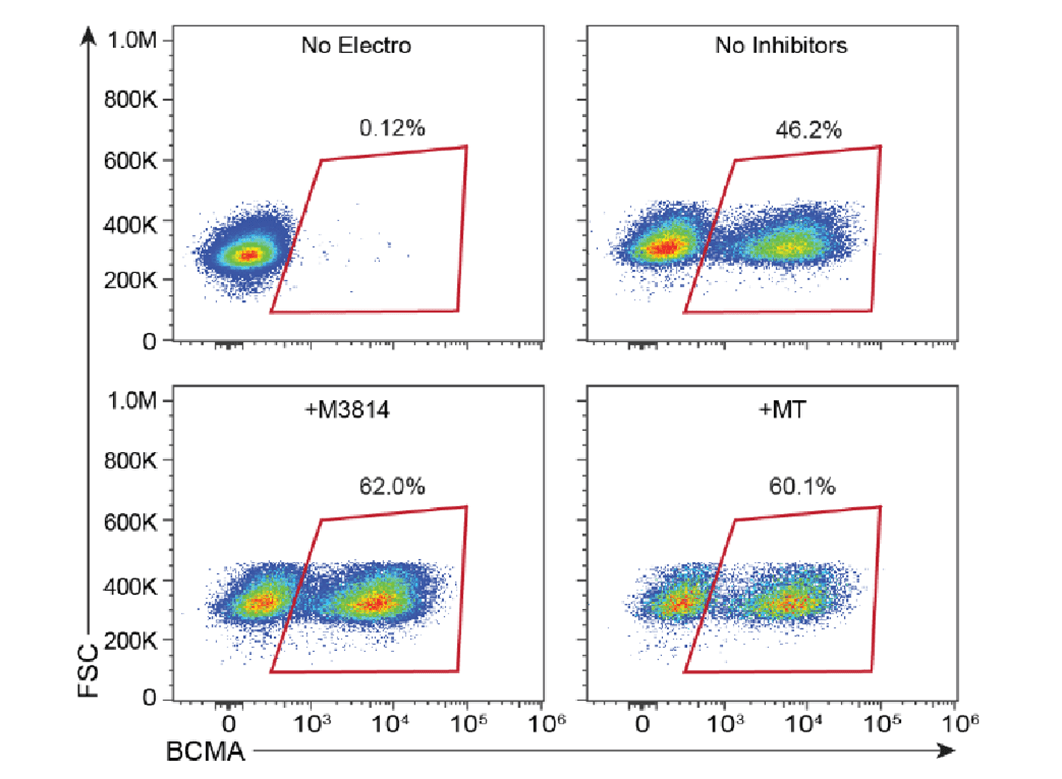
High knockin efficiency at the TRAC locus with MaxCyte electroporation.
This content was reproduced with the kind permission of Alexander Marson, bioRxiv 2021.09.02.458799.
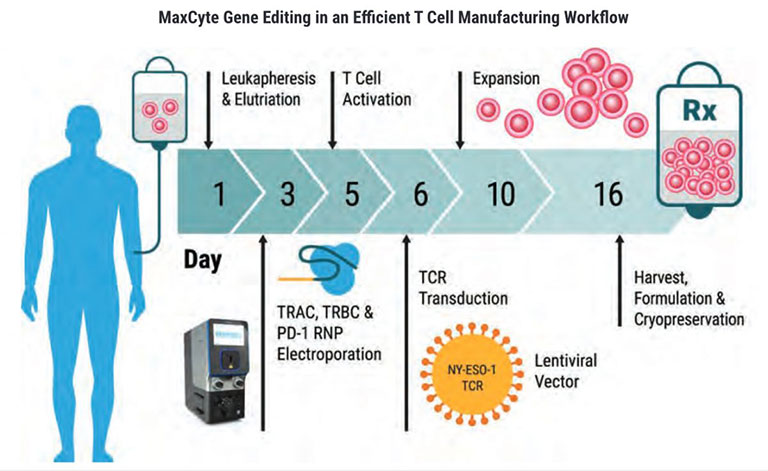
Enable highly efficient targeted knockin
The scalability of the MaxCyte GTx® system eliminates experimental re-optimization ensuring seamless CAR T cell development from initial research to commercialization. Moreover, the GTxTM enables highly efficient transfection of large cell populations, ensuring consistent and high-quality CAR T cell production.
Efficient integration of CAR genes into targeted sites such as the T Cell Receptor Alpha Constant (TRAC) locus has proved challenging even with precision genome engineering tools. MaxCyte enables delivery of diverse payloads resulting in highly efficient integration. Discover effortless CAR T cell engineering with MaxCyte.
Achieve seamless scalability
Although the development of CAR T cell therapies hold promise for cancer treatment, this approach is fraught with obstacles such as extended development timelines and costly manufacturing. Explore how MaxCyte enabled the cGMP manufacture of stable CAR T cells with enhanced anti-tumor activity in only five days.
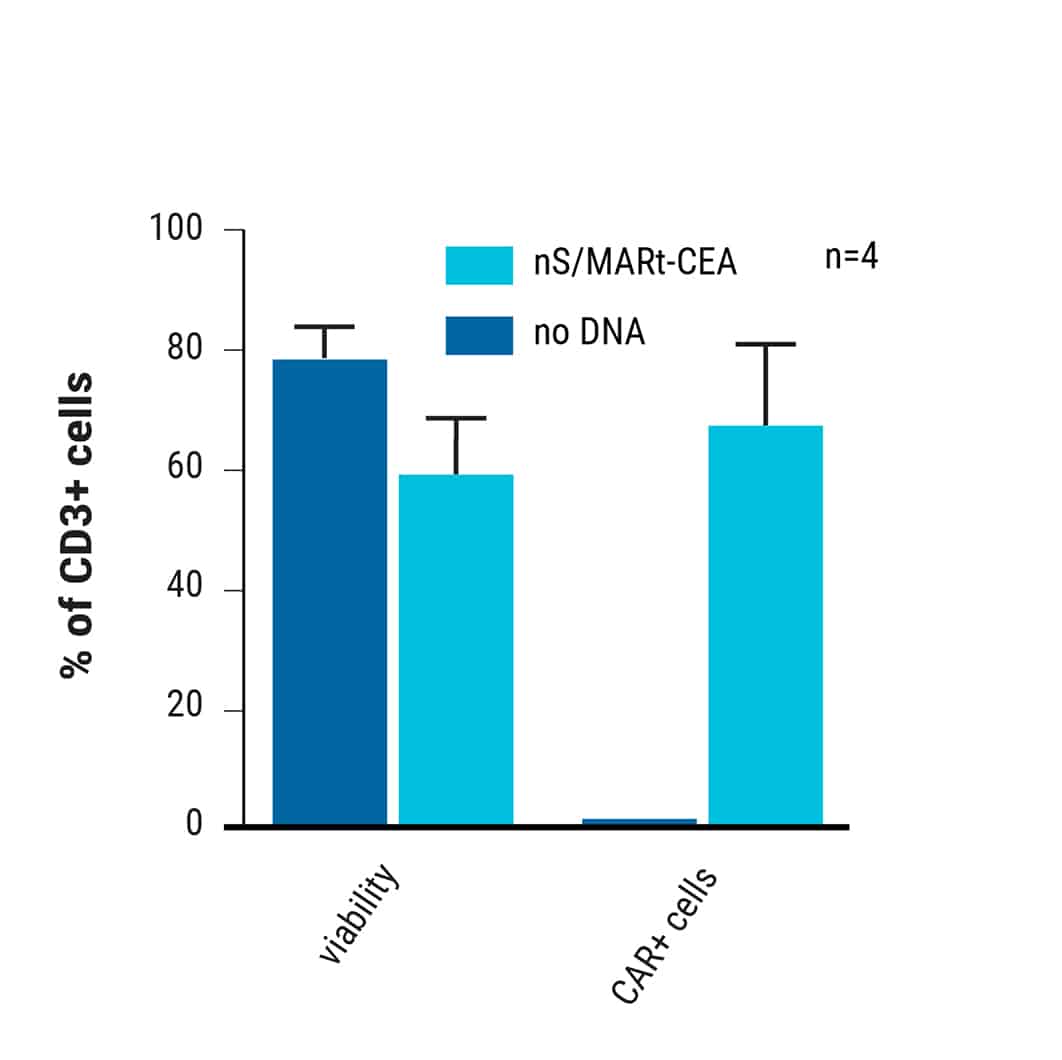
nS/MARt vector expressing the CEA-CAR delivered with high efficacy and viability into activated human CD3+ cells.
This content was reproduced with the kind permission of Richard Harbottle.
CAR T cell manufacturing success with MaxCyte
Genetically engineering T cells to express CARs that recognize tumor-associated antigens is an approach that holds immense potential for treating cancer. Chart a course to success by partnering with us to navigate development and manufacturing challenges.
Take a deeper dive into specific cell therapy development applications
See examples of real-world data for each cell type and find out how MaxCyte can help you chart a clear path from concept to the clinic.
Working with other cell types?
Related resources
ExPERT GTx® electroporation system
The ExPERT GTx® is the only non-viral transfection system enabling a commercially approved cell therapy. Clinically-proven in over 45 clinical trials across various indications and gene editing tools, our ExPERT GTx® system and on-demand scientific support helps accelerating development while mitigating risks.
Benefit from our proven track record of reducing preclinical development by multiple months with our scientific knowhow that extends beyond the electroporation step. Embrace continuous scalability with the only platform offering transition from 15uL to 100mL on a single instrument.


Reagents and processing assemblies
MaxCyte's consumable products provide users with a variety of options for project scale and throughput from discovery through cGMP manufacturing using a single platform. Our range of Processing Assemblies allows users to transfect a variety of cell sample volumes to meet specific application needs. MaxCyte's Electroporation Buffer is animal-derived component free and safe for all cell types ensuring consistent, high-performance transfection.
Ready to learn more about our technology?
Find out how the ExPERT platform can accelerate your CAR T cell manufacturing journey.