TCR-T Cell Therapy
Whether you want to express exogenous T cell receptor (TCR) mRNA or to knock out endogenous TCR with CRISPR RNP, MaxCyte® ExPERTTM instruments can streamline your journey to success with reliable, high-efficiency electroporation and high cell viability.
Introduction
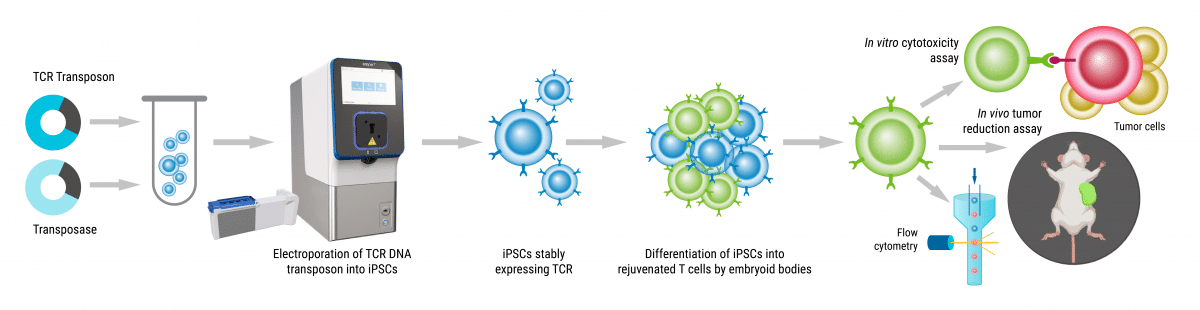
The development of TCR-T cell therapy has transformed the way we approach treating cancer. In this innovative strategy, engineered TCRs are introduced to the patient's T cells through viral or non-viral gene transfer techniques. This genetic modification enables the T cells to recognize cancer cells and attach to them.
After the genetic modification, the TCR-T cells undergo expansion and rigorous quality control testing to ensure their potency, purity, and safety. As with any new technology, TCR-T cell manufacturing faces numerous challenges from efficient delivery of diverse payloads to scale-up of final therapeutic material.
Explore multiplex engineering
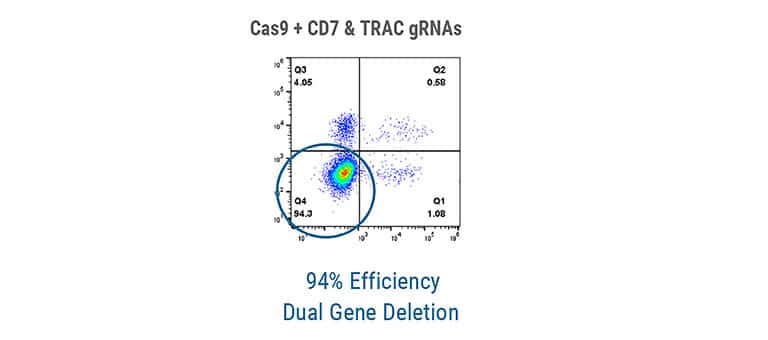
Designing potent, safe and persistent T cell therapies requires sophisticated engineering strategies, which can be challenging to implement, particularly at the GMP scale necessary for the clinic. Explore how MaxCyte enabled the first-ever human clinical trial of multiplex CRISPR gene-edited T cell therapy.
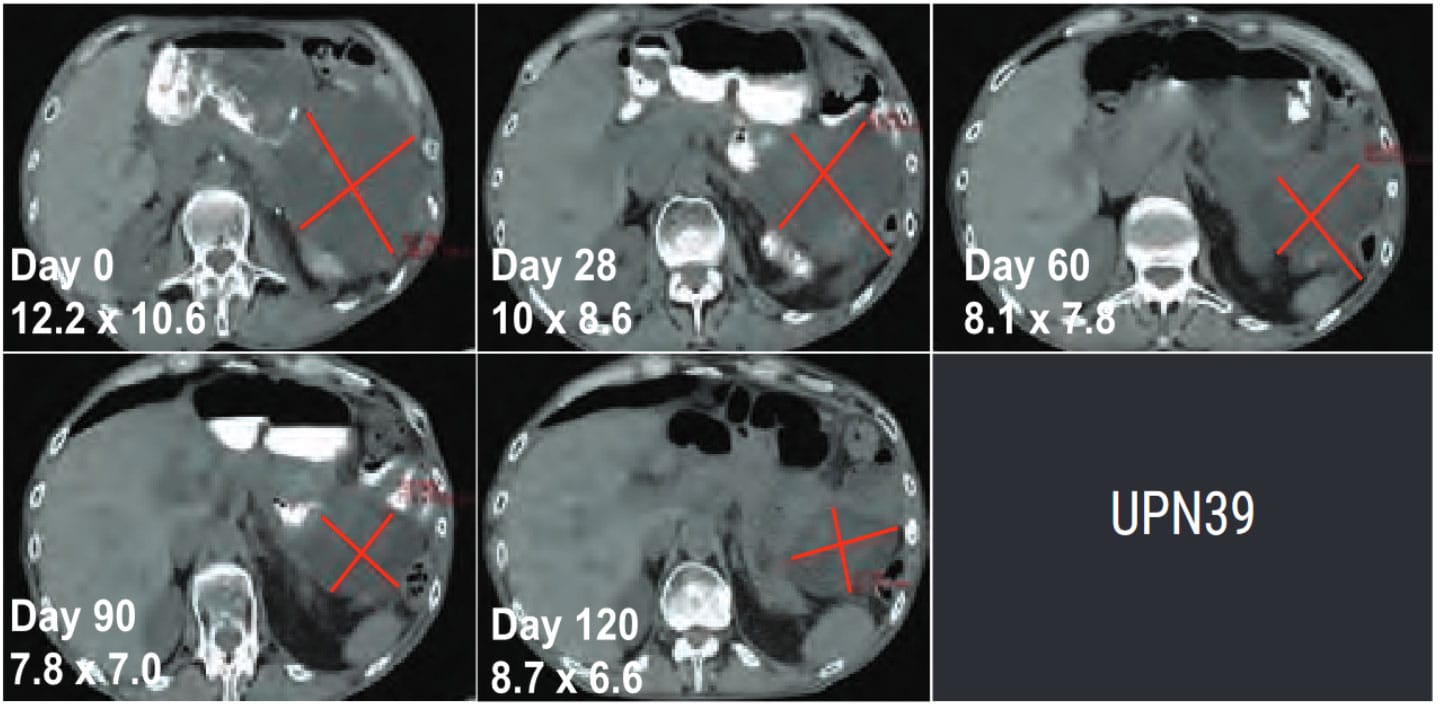
CT Scans show 50% tumor regression after infusion with TCR immunotherapy.
Adapted from Carl June Science, 2020, 367(6481):eaba7365.
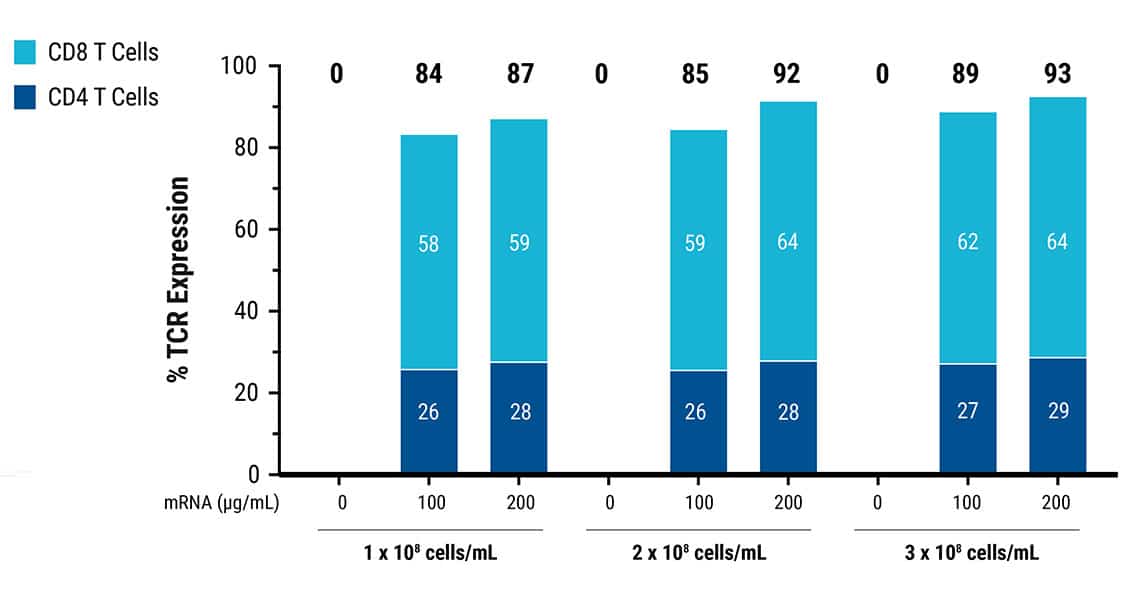
TCR expression was achieved in 84-93% of T cells.
This content was reproduced with the kind permission of Lion TCR.
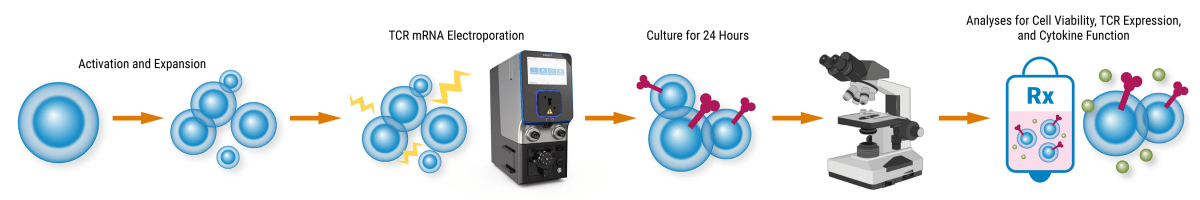
Express exogenous TCR mRNA
Engineering T cells to express new TCRs can be challenging. Timelines, cost and potential toxicity are all factors that must be balanced when designing new cell therapies. MaxCyte electroporation offers a high-efficiency, scalable, cost-effective and safe approach to delivering a variety of gene editing tools, including exogenous mRNA for optimized T cell manufacturing.
Generating TCR-T cells is rapid, scalable and clinically feasible
Steer your way to a successful T cell therapy by partnering with MaxCyte. For sophisticated multiplexed engineering and highly efficient delivery of a variety of payloads, we are here to support your discovery from concept to commercialization.
Take a deeper dive into specific cell therapy development applications
See examples of real-world data for each cell type and find out how MaxCyte can help you chart a clear path from concept to the clinic.
Working with other cell types?
Resources
ExPERT GTx® electroporation system
The ExPERT GTx® is the only non-viral transfection system enabling a commercially approved cell therapy. Clinically-proven in over 45 clinical trials across various indications and gene editing tools, our ExPERT GTx® system and on-demand scientific support helps accelerating development while mitigating risks.
Benefit from our proven track record of reducing preclinical development by multiple months with our scientific knowhow that extends beyond the electroporation step. Embrace continuous scalability with the only platform offering transition from 15uL to 100mL on a single instrument.


Reagents and processing assemblies
MaxCyte's consumable products provide users with a variety of options for project scale and throughput from discovery through cGMP manufacturing using a single platform. Our range of Processing Assemblies allows users to transfect a variety of cell sample volumes to meet specific application needs. MaxCyte's Electroporation Buffer is animal-derived component free and safe for all cell types ensuring consistent, high-performance transfection.
Ready to learn how to advance your T cell engineering projects?
Our experienced team is here to help.